- Department of Neurosurgery, Hospital da Restauração,
- Department of Neurosurgery, Real Hospital Português de Beneficência em Pernambuco,
- Department of Medicine, Faculty of Medical Science, University of Pernambuco, Recife, Brazil.
Correspondence Address:
João Ribeiro Memória
Department of Neurosurgery, Hospital da Restauração,
DOI:10.25259/SNI_321_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: João Ribeiro Memória, Erlan Pércio Lopes Rufino, Pedro Lukas do Rêgo Aquino, Francisco Vaz Guimarães Filho, Túlio Maranhão Neto, Herika Karla Negri Brito de Vasconcelos. Brain aspergilloma in an immunocompetent individual: A case report. 25-Jul-2020;11:211
How to cite this URL: João Ribeiro Memória, Erlan Pércio Lopes Rufino, Pedro Lukas do Rêgo Aquino, Francisco Vaz Guimarães Filho, Túlio Maranhão Neto, Herika Karla Negri Brito de Vasconcelos. Brain aspergilloma in an immunocompetent individual: A case report. 25-Jul-2020;11:211. Available from: https://surgicalneurologyint.com/surgicalint-articles/10153/
Abstract
Background: Aspergillosis is caused by fungi from the Aspergillus species, mainly Aspergillus fumigatus. Patients with aspergillosis typically have an underlying immunosuppression, and it is rare within the central nervous system (CNS) in an immunocompetent host. The mortality rate is as high as 90% if untreated, and the diagnosis is usually delayed due to nonspecific clinical presentation. This study described a case of CNS aspergillosis in an immunocompetent patient, through which we sought to discuss the current knowledge regarding brain Aspergillus focusing in the immunocompetent host.
Case Description: A 45-year-old male presented with 2 years of low-intensity headache and history of chronic sinusitis with epistaxis in the left nostril. Fifteen days before admission, he had high-intensity headache, episodes of transient aphasia, and seizure. Imaging showed a contrast-enhancing mass within the left maxillary sinus and another lesion in the left frontal lobe. The left frontal craniotomy was conducted, and complete resection was achieved. Biopsy identified A. fumigatus, and antifungal therapy was initiated. After 2 weeks, a new lesion was detected in the right frontal lobe, and the patient underwent a new procedure with complete lesion resection. Follow-up at 3 weeks did not reveal any evidence of residual or recurrent disease. The patient did not develop neurological complaints and was referred for resection of the remaining lesion by an otolaryngology team.
Conclusion: Being one of the few cases reporting a successful outcome for brain aspergilloma in an immunocompetent patient after complete surgical resection and amphotericin B and itraconazole therapy, we sought to reveal novel insight into brain aspergillosis.
Keywords: Aspergillosis, Central nervous system, Fungal infection, Immunocompetent host, Surgery
INTRODUCION
Aspergillosis is caused by ubiquitous and saprophytic fungi of the Aspergillus species. The most common human pathogen is Aspergillus fumigatus.[
We reported a rare case of CNS aspergillosis in an immunocompetent patient, and this article sought to reveal the current knowledge regarding brain Aspergillus focusing on the immunocompetent host.
CASE REPORT
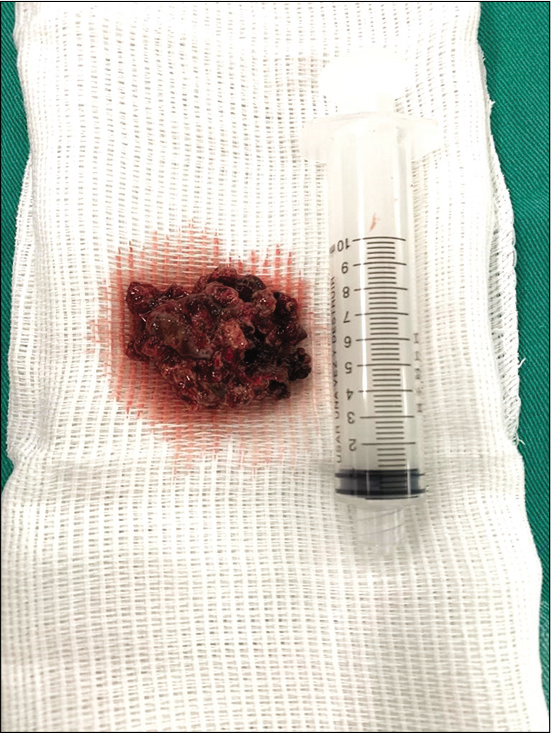
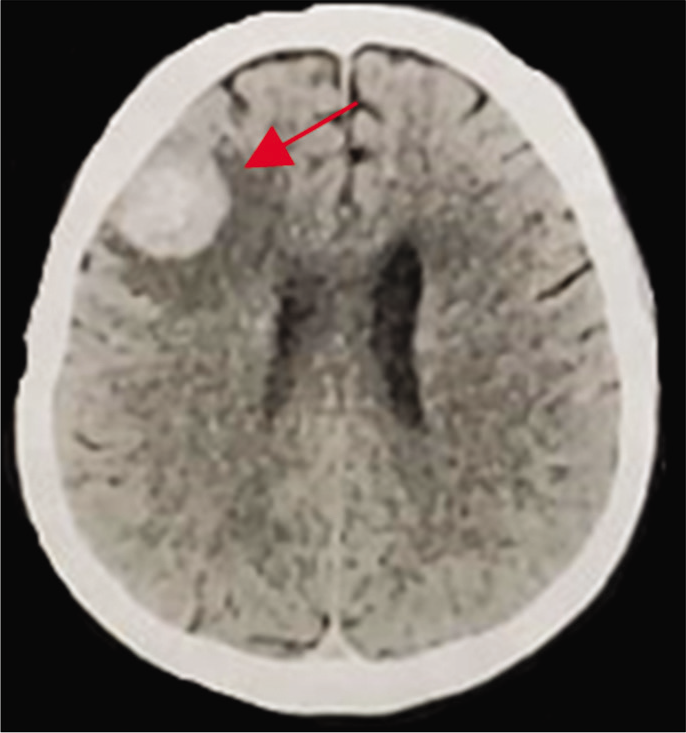
A 45-year-old male presented with 2 years of low- intensity headache and history of chronic sinusitis with epistaxis in the left nostril. Fifteen days before admission, he had high-intensity headache, episodes of transient aphasia, and seizure episodes. He was alert, afebrile, and hemodynamically stable, and his neurological examination was unremarkable. His personal history includes cocaine use and smoking. He did not have diabetes mellitus, HIV infection, or any previous chemotherapy or immunomodulatory therapies. There was no history of craniofacial trauma. There were not regular medications. Routine investigations, including full blood count, serum electrolytes, and C-reactive protein, were normal. A computed tomography (CT) demonstrated a hyperdense mass in the left frontal region. Magnetic resonance imaging (MRI) revealed a contrast-enhancing mass within the left maxillary sinus measuring 51 × 30 × 14 mm [
DISCUSSION AND SHORT LITERATURE REVIEW
Physiopathology
CNS manifestations of intracranial aspergillosis include intracranial aneurysms, meningitis, infarction, hemorrhage, and space-occupying granulomas.[
Clinical features
The most common presentation of CNS aspergillosis includes meningitis, abscess, cerebritis, infarction, mycotic aneurysms, and granuloma.[
Brain aspergillosis in immunocompetent hosts has been reported mainly from African countries.[
Clinical presentations are similar to those in neoplasms, making diagnosis problematic. The most common symptoms involved focal neurologic deficits, and it depends on the region affected.[
Diagnosis
The clinical diagnosis is difficult to make because the presenting symptoms and signs are nonspecific. Radiologically, differential diagnosis can be made with abscess, brain tumor, tuberculosis, or hemorrhage. In our review, the most common differential diagnosis in the immunocompetent host was tuberculosis, following to brain tumor.[
On CT scan, the lesion commonly appears hyperdense with calcification, and, in most of the cases, mass effect signs may be visualized.[
MRI images show irregular space-occupying lesion from iso-to-hypointense. On T1 postgadolinium, there is a bright homogenous ring enhancement due to necrosis, fungal, and vascular involvement. Hypointensity on T2-weighted images is an important point in the aspergilloma diagnosis, indicating high concentrations of ferromagnetic elements, including iron, zinc, and magnesium – critical for fungal amino acid metabolism.[
The diagnosis can be confirmed only through histopathological examination or culture of biopsy the specimen.[
Treatment and prognosis
Immune status is crucial to determine the clinical outcomes.[
The biopsy may be performed through stereotactic or craniectomy. Stereotactic biopsy is associated with higher disease recurrence.[
CONCLUSION
To the best of our knowledge, this is one of the few cases reported of successful treatment of brain aspergilloma in an immunocompetent patient with amphotericin B and itraconazole after complete surgical resection. However, longer follow-up is needed. Our case illustrates the importance of radical surgical resection and antifungal therapy in the treatment of these cases. This article also sought to reveal novel insight into brain aspergillosis in immunocompetent hosts.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahmadzai H, Raley DA, Masters L, Davies M. An unusual case of a pituitary fossa aspergilloma in an immunocompetent patient mimicking infiltrative tumour. J Surg Case Rep. 2013. 4: 1-4
2. Alahmari AF. Medical treatment of brain aspergilloma followed by MRI: A case report. Radiol Case Rep. 2019. 14: 103-11
3. Alrajhi AA, Enani M, Mahasin Z, Al-Omran K. Chronic invasive aspergillosis of the paranasal sinuses in immunocompetent hosts from Saudi Arabia. Am J Trop Med Hyg. 2001. 65: 83-6
4. Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996. 23: 608-15
5. Elgamal EA, Murshid WR. Intracavitary administration of amphotericin B in the treatment of cerebral aspergillosis in a non-immune-compromised patient: Case report and review of the literature. Br J Neurosurg. 2000. 14: 137-41
6. Ellenbogen JR, Waqar M, Cooke RP, Javadpour M. Management of granulomatous cerebral aspergillosis in immunocompetent adult patients: A review. Br J Neurosurg. 2016. 30: 280-5
7. Espinel-Ingroff A. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J Clin Microbiol. 2001. 39: 954-8
8. Harris DE, Enterline DS. Neuroimaging of AIDS. I. Fungal infections of the central nervous system. Neuroimaging Clin N Am. 1997. 7: 187-98
9. Hussain S, Salahuddin N, Ahmad I, Salahuddin I, Jooma R. Rhinocerebral invasive mycosis: Occurrence in immunocompetent individuals. Eur J Radiol. 1995. 20: 151-5
10. Isselbacher KJ, Braunwald E, Wilson JD, Isselbacher KJ, Braunwald E, Wilson JD.editors. Harrison’s Principles of Internal Medicine. New York: Mc Graw Hill; 1995. p. 855-2
11. Kim DG, Hong SC, Kim HJ, Chi JG, Han MH, Choi KS. Cerebral aspergillosis in immunologically competent patients. Surg Neurol. 1993. 40: 326-31
12. Koshy R, Malhotra P. Treatment of primary aspergilloma of the central nervous system in a diabetic immunocompetent patient with surgical resection and voriconazole: A case report and review of the literature. Turk Neurosurg. 2011. 21: 641-4
13. Kumar D, Nepal P, Singh S, Ramanathan S, Khanna M, Sheoran R. CNS aspergilloma mimicking tumors: Review of CNS aspergillus infection imaging characteristics in the immunocompetent population. J Neuroradiol. 2018. 45: 169-76
14. Nabika S, Kiya K, Satoh H, Mizoue T, Araki H, Oshita J. Local administration of amphotericin B against aspergilloma in the prepontine cistern-case report. Neurol Med Chir. 2007. 47: 89-92
15. Nadkarni TD, Desai KI, Muzumdar D. Ischaemic complications after surgical resection of intracranial aspergilloma. J Clin Neurosci. 2003. 10: 500-2
16. Sahip NB, Santral DB. Treatment of primary aspergilloma of the central nervous system in a diabetic immunocompetent patient with surgical resection and voriconazole: A case report and review of the literature. Turk Neurosurg. 2011. 21: 641-4
17. Scully EP, Baden LR, Katz JT. Fungal brain infections. Curr Opin Neurol. 2008. 21: 347-52
18. Segal BH. Aspergillosis. N Engl J Med. 2009. 360: 1870-84
19. Siddiqui AA, Shah AA, Bashir SH. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: Clinical spectrum and outcome in 25 cases. Neurosurgery. 2004. 55: 602-13
20. Steinbach WJ, Stevens DA, Denning DA. Combination and sequential antifungal therapy for invasive aspergillosis: Review of published in vitro and in vivo interactions and 6281 clinical cases from 1966 to 2001. Clin Infect Dis. 2003. 37: 188-224
21. Xiao A, Jiang S, Liu Y, Deng K, You C. Invasive intracranial aspergillosis spread by the pterygopalatine fossa in an immunocompetent patient. Braz J Infect Dis. 2012. 16: 192-5
22. Yamada K, Shrier DA, Rubio A, Shan Y, Zoarski GH, Yoshiura T. Imaging findings in intracranial aspergillosis. Acad Radiol. 2002. 9: 163-71