- Department of Neurosurgery, Kobe University Graduate School of Medicine, Kobe, Japan
Correspondence Address:
Hiroki Goto, Department of Neurosurgery, Kobe University Graduate School of Medicine, Kobe, Japan.
DOI:10.25259/SNI_637_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Goto, Atsushi Fujita, Naoto Nakamura, Masaaki Kohta, Takashi Sasayama. Brainstem congestion as the initial presentation of cavernous sinus dural arteriovenous fistula without ocular symptoms. 04-Oct-2024;15:359
How to cite this URL: Hiroki Goto, Atsushi Fujita, Naoto Nakamura, Masaaki Kohta, Takashi Sasayama. Brainstem congestion as the initial presentation of cavernous sinus dural arteriovenous fistula without ocular symptoms. 04-Oct-2024;15:359. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13133
Abstract
Background: Cavernous sinus dural arteriovenous fistula (CSDAVF) is an abnormal arteriovenous connection involving the dura mater within or adjacent to the wall of the cavernous sinus. While cases with superior ophthalmic vein drainage and ocular symptoms are typical, we report a rare case of CSDAVF draining into the perimedullary vein of the medulla oblongata and spinal cord and causing cerebellar ataxia and myelopathy as the initial presentation.
Case Description: A 73-year-old man presented with vertigo and rapidly progressing gait disturbance. Digital subtraction angiography revealed a left CSDAVF draining only into the spinal perimedullary veins, which was classified as Cognard type V. We performed a transvenous embolization through the occluded left inferior petrosal sinus and achieved a super-selective shunt occlusion using three platinum coils. The postoperative course was uneventful with immediate improvement of symptoms.
Conclusion: CSDAVF should be considered as a differential diagnosis in a patient with venous congestion in the brainstem.
Keywords: Brainstem, Cavernous sinus, Dural arteriovenous fistula, Myelopathy, Venous congestion
INTRODUCTION
Cavernous sinus dural arteriovenous fistula (CSDAVF) is an abnormal arteriovenous connection involving the dura mater within or adjacent to the wall of the cavernous sinus. The clinical presentation depends on the pattern of venous drainage. When the drainage is diverted anteriorly into the superior ophthalmic vein, it causes ocular symptoms such as chemosis, proptosis, and ophthalmoplegia. Posterior drainage through the inferior petrosal sinus may cause pulsatile tinnitus, while critical venous infarction or hemorrhage may, in rare cases, result from cortical venous reflux through the superficial middle Sylvian, uncal, or petrosal veins.[
Reports of CSDAVF presenting with symptoms attributed to brainstem or spinal cord congestion and without ocular symptoms are rare. This report describes a rare case of CSDAVF draining into the perimedullary vein of the medulla oblongata and spinal cord alone, which caused cerebellar ataxia and myelopathy without any ocular symptoms.
CASE REPORT
A 73-year-old man presented with vertigo and rapidly progressive gait disturbance. He had a previous medical history of cholecystectomy, hypertension, and dysuria and reported the onset of dysphagia 4 months earlier. Three months later, he developed nausea and vertigo followed by progressive left side-dominant paraparesis and bilateral lower limb paresthesia. Gait disturbance progressed rapidly within 2 weeks, and eventually, he required a wheelchair for daily activities. T2-weighted magnetic resonance imaging (MRI) showed an area of high-signal intensity in the medulla oblongata and the upper cervical spinal cord. Multiple flow voids were detected around the brainstem [
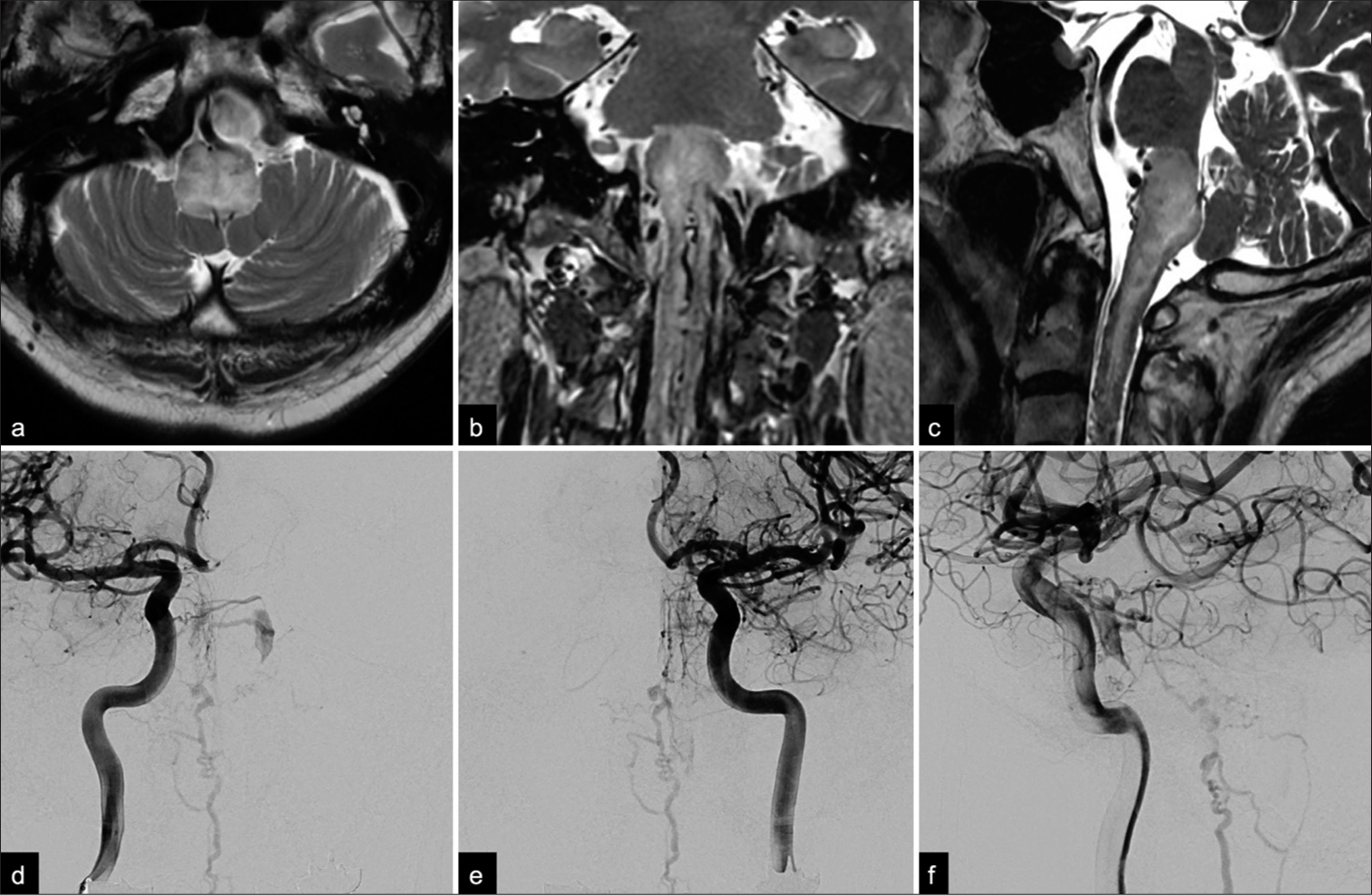
Figure 1:
Preoperative (a) axial, (b) coronal, and (c) sagittal T2-weighted magnetic resonance images showing an area of high-signal intensity in the medulla oblongata and upper cervical spinal cord. Note that multiple flow void signs were detected on both the dorsal and ventral aspects of the brainstem. Anterior-posterior angiographic views of the (d) right and (e) left internal carotid arteries show a small opacification of the left cavernous sinus draining into the anterior pontomesencephalic vein through the pontine bridging vein. (f) A late arterial phase lateral view on a left internal carotid angiogram showed opacification of the posterior part of the cavernous sinus and tortuous congested veins around the medulla oblongata.
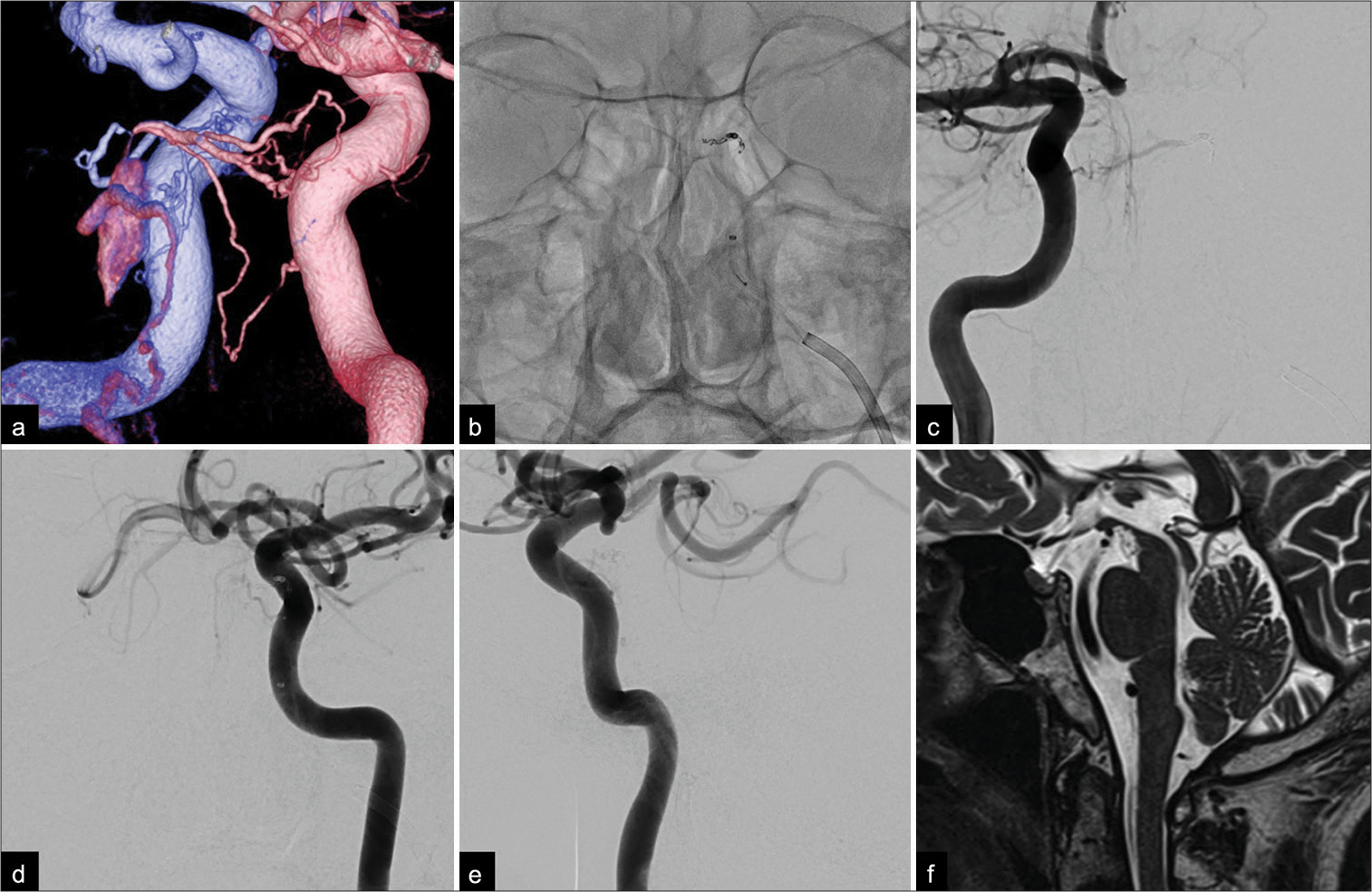
Figure 2:
(a) Posterior-anterior oblique view of a fused bilateral internal carotid three-dimensional digital subtraction angiogram showing the shunting point, which consisted of the left inferolateral trunk (blue) and the right meningohypophyseal trunk (pink). Note the small opacification in the posterior part of the cavernous sinus and the pontine bridging vein draining into the pontomesencephalic vein. (b) Anterior-posterior view on a non-subtracted craniogram obtained during embolization showed coils placed at the feeder as well as the shunting point. Postoperative anterior-posterior angiograms of both internal carotid arteries ((c) right; (d) left) and a (e) lateral angiogram of the left internal carotid artery showing complete obliteration of the fistula and the tortuous veins around the medulla oblongata. (f) A sagittal T2-weighted magnetic resonance image obtained 6 months after treatment showed a complete disappearance of the area of high signal intensity and flow voids around the brainstem and spinal cord.
DISCUSSION
Symptoms of CSDAVF are thought to be associated with the type of drainage route, and retrograde cortical venous drainage into the superficial middle cerebral, basal vein, and posterior fossa has the potential to produce aggressive symptoms.[
CSDAVF causing venous congestion of the medulla oblongata and the spinal cord is rare, and very few cases have been reported. A review of 100 patients with progressive myelopathy caused by a Cognard type V dural arteriovenous fistula identified only 1 case of CSDAVF.[
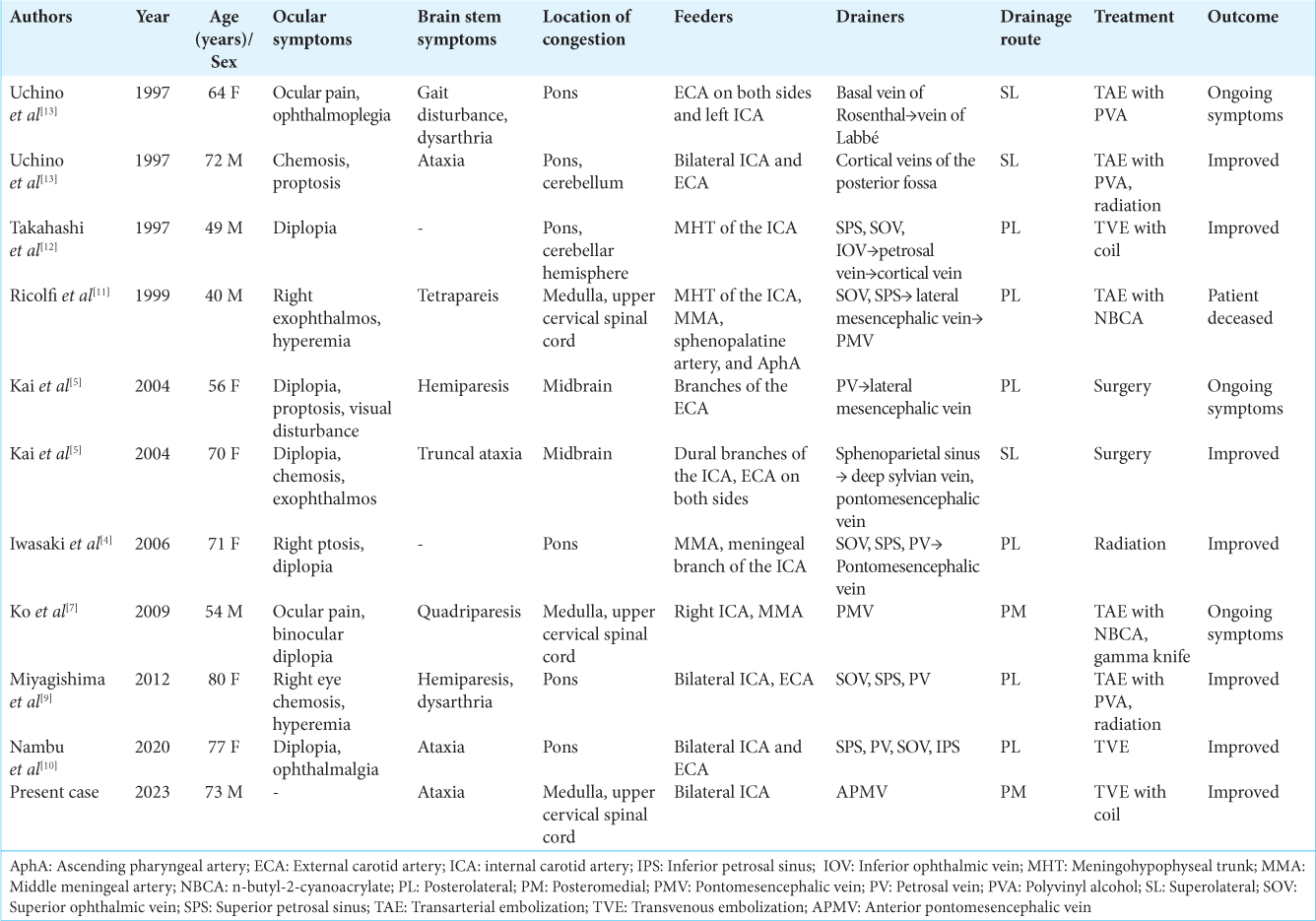
We conducted a systematic search for patients who had CSDAVF associated with brainstem edema and identified 10 cases (11, including our present case) with sufficient angiographic data for evaluation of the drainage route[
CONCLUSION
Unlike typical cases of CSDAVF, our patient presented with symptoms attributable to venous congestion of the medulla oblongata without any ocular symptoms. CSDAVF should be borne in mind as a differential diagnosis in a patient with venous congestion in the brainstem.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Awad IA, Little JR, Akrawi WP, Ahl J. Intracranial dural arteriovenous malformations: Factors predisposing to an aggressive neurological course. J Neurosurg. 1990. 72: 839-50
2. De Grado A, Manfredi C, Brugnera A, Groppo E, Valvassori L, Cencini F. Watch brain circulation in unexplained progressive myelopathy: A review of Cognard type V arteriovenous fistulas. Neurol Sci. 2023. 44: 3457-80
3. Hou K, Li G, Qu L, Liu H, Xu K, Yu J. Intracranial dural arteriovenous fistulas with brainstem engorgement: An under-recognized entity in diagnosis and treatment. Front Neurol. 2020. 11: 526550
4. Iwasaki M, Murakami K, Tomita T, Numagami Y, Nishijima M. Cavernous sinus dural arteriovenous fistula complicated by pontine venous congestion. A case report. Surg Neurol. 2006. 65: 516-8
5. Kai Y, Hamada JI, Morioka M, Yano S, Ushio Y. Brain stem venous congestion due to dural arteriovenous fistulas of the cavernous sinus. Acta Neurochir (Wien). 2004. 146: 1107-11
6. Kiyosue H, Mori H, Sagara Y, Hori Y, Okahara M, Nagatomi H. Basal cerebral venous drainage from cavernous sinus dural arteriovenous fistulas. Neuroradiology. 2009. 51: 175-81
7. Ko SB, Kim CK, Lee SH, Yoon BW. Carotid cavernous fistula with cervical myelopathy. J Clin Neurosci. 2009. 16: 1350-3
8. Lasjaunias P, Chiu M, Ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986. 64: 724-30
9. Miyagishima T, Hara T, Inoue M, Terano N, Ohno H, Okamoto K. Pontine venous congestion due to dural arteriovenous fistula of the cavernous sinus: Case report and review of the literature. Surg Neurol Int. 2012. 3: 53
10. Nambu K, Misaki K, Yoshikawa A, Kamide T, Nambu I, Sasagawa Y. Cavernous sinus dural arteriovenous fistula with an enhanced lesion in the brainstem mimicking a malignant tumor. World Neurosurg. 2020. 140: 13-7
11. Ricolfi F, Manelfe C, Meder JF, Arrué P, Decq P, Brugiéres P. Intracranial dural arteriovenous fistulae with perimedullary venous drainage. Anatomical, clinical and therapeutic considerations. Neuroradiology. 1999. 41: 803-12
12. Takahashi S, Tomura N, Watarai J, Mizoi K, Manabe H. Dural arteriovenous fistula of the cavernous sinus with venous congestion of the brain stem: Report of two cases from the Departments of Radiology. AJNR Am J Neuroradiol. 1999. 20: 886-8
13. Uchino A, Kato A, Kuroda Y, Shimokawa S, Kudo S. Pontine venous congestion caused by dural carotid-cavernous fistula: Report of two cases. Eur Radiol. 1997. 7: 405-8
14. Viñuela F, Fox AJ, Debrun GM, Peerless SJ, Drake CG. Spontaneous carotid-cavernous fistulas: Clinical, radiological, and therapeutic considerations: Experience with 20 cases. J Neurosurg. 1984. 60: 976-84