- Department of Neurosurgery, Jacobs School of Medicine and Biomedical Sciences, State University of New York, University at Buffalo, Buffalo, New York, USA
- Department of Neurosurgery, Kaleida Health, Gates Vascular Institute, Buffalo General Medical Center, University at Buffalo, Buffalo, New York, USA
- Department of Pathology, Kaleida Health, Buffalo General Medical Center, State University of New York, University at Buffalo, Buffalo, New York, USA
- Department of Neurosurgery, Department of Medical Imaging, Division of Surgery, University of Arizona, Tucson, Arizona, USA
- Department of Radiology, Jacobs School of Medicine and Biomedical Sciences, State University of New York, University at Buffalo, Buffalo, New York, USA
- Toshiba Stroke and Vascular Research Center, State University of New York, University at Buffalo, Buffalo, New York, USA
- Jacobs Institute, Buffalo, New York, USA
Correspondence Address:
Adnan H. Siddiqui
Department of Neurosurgery, Jacobs School of Medicine and Biomedical Sciences, State University of New York, University at Buffalo, Buffalo, New York, USA
Department of Neurosurgery, Kaleida Health, Gates Vascular Institute, Buffalo General Medical Center, University at Buffalo, Buffalo, New York, USA
Department of Radiology, Jacobs School of Medicine and Biomedical Sciences, State University of New York, University at Buffalo, Buffalo, New York, USA
Toshiba Stroke and Vascular Research Center, State University of New York, University at Buffalo, Buffalo, New York, USA
Jacobs Institute, Buffalo, New York, USA
DOI:10.4103/2152-7806.171239
Copyright: © 2015 Shakir HJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Shakir HJ, Diletti SM, Hart AM, Meyers JE, Dumont TM, Siddiqui AH. Carotid body tumor imitator: An interesting case of Castleman's disease. Surg Neurol Int 07-Dec-2015;6:181
How to cite this URL: Shakir HJ, Diletti SM, Hart AM, Meyers JE, Dumont TM, Siddiqui AH. Carotid body tumor imitator: An interesting case of Castleman's disease. Surg Neurol Int 07-Dec-2015;6:181. Available from: http://surgicalneurologyint.com/surgicalint_articles/carotid-body-tumor-imitator-an-interesting-case-of-castlemans-disease/
Abstract
Background:There are very few reports in the literature of Castleman's disease affecting the carotid artery and a single previous report of a case of Castleman's disease of the neck originally mistaken as a carotid body tumor.
Case Description:We describe a rare case of Castleman's disease, manifesting with classic radiographic hallmarks of a carotid body tumor. The postoperative pathologic examination identified the resected mass as Castleman's lymphadenopathy. The management of this particular case is discussed, and the findings are highlighted.
Conclusions:We present a unique case of a tumor initially and incorrectly diagnosed as a carotid body tumor. However, after comprehensive treatment with endovascular and surgical modalities and subsequent pathologic examination, the diagnosis of this rare entity was made.
Keywords: Carotid body tumor, Castleman's disease, lymphadenopathy, paraganglioma
INTRODUCTION
Carotid body tumors, typically found at the carotid bifurcation, are rare entities among head and neck neoplasms. Most (60–70%) head and neck paragangliomas occur in the region of the carotid bifurcation.[
The patient described in this report presented with a nonsecretory tumor causing widening of the carotid bifurcation. Although observation for small, nonsecreting tumors may represent an option, typically surgical intervention is required for cases involving the carotid artery. Without intervention, progressive symptomatology due to damage of the neighboring vagus and hypoglossal nerves in addition to compromise of the integrity of the carotid artery itself should be expected.[
CASE REPORT
Examination
A 46-year-old African-American man presented with an enlarging, tender, left-sided neck mass. The initial evaluation consisted of magnetic resonance and computed tomographic (CT) imaging that demonstrated a 7-cm mass with T1 signal and T2 signal contrast enhancement [
Figure 1
Axial T1-weighted magnetic resonance images without (a) and with (b) gadolinium demonstrating a 7-cm homogeneously enhancing left neck mass circumferentially encasing the left internal carotid artery, extending from C2 to C4. Sagittal (c) and axial (d) T2-weighted magnetic resonance images, again showing the left neck mass encasing the left internal carotid artery
After informed consent was obtained, digital subtraction angiography and balloon occlusion testing were performed. Angiography confirmed a 7-cm vascular tumor with very thin vascular channels arising directly from the walls of the common and internal carotid arteries. There was no pedicle large enough to be suitable for embolization [
Operation
Surgical exposure of the carotid sheath revealed a large mass in the region of the carotid bifurcation. Exposure from the skull base to the omohyoid muscle allowed sequential separation of the jugular vein and the vagus, accessory, and hypoglossal nerves from the mass. The entire carotid bifurcation was dilated without separation of planes between the internal and external carotid arteries, carotid bulb, and tumor. The common, internal, and external carotid arteries were subsequently ligated away from the tumor; and the tumor and neighboring arterial segments were resected en bloc and sent for pathologic examination. Four distinctive lymph nodes appreciated during neck dissection were additionally submitted for examination. After resection of the mass, the sympathetic chain ganglia below the carotid sheath became apparent and appeared uninvolved.
Postoperative course
The patient woke up without deficits; however, on the second postoperative day, he developed mild hoarseness and swallowing difficulty with an enlarging neck mass. He was taken emergently for the evacuation of a neck hematoma. No active bleeding sites were noted. Thereafter, the patient made an uneventful recovery and was discharged home without evidence of cranial neuropathies.
Pathological findings
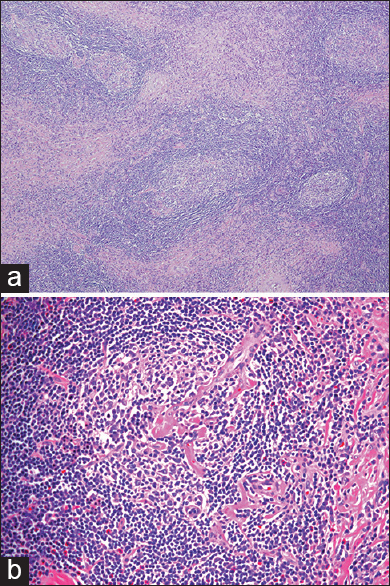
Gross examination of the en bloc specimen revealed a segment of artery surrounded by a mass measuring 4.8 cm × 4.0 cm × 2.4 cm. The tumor had a tan, myxoid surface with yellow tissue, and hemorrhagic changes next to the thrombosed arterial segment. Microscopic examination revealed a large circumscribed cervical lymph node with a large elastic artery coursing through it. The lymph node showed distinctive features including follicles composed of concentric rings of mantle zone lymphocytes around germinal centers as well as prominent vascularity of the germinal centers, often showing a single penetrating arteriole [Figure
Figure 3
(a) Hematoxylin and eosin (H and E) stain at low-power magnification (×40) demonstrating lymph node hyperplasia with vascular proliferation magnification. (b) H and E stain at high-power magnification (×200) showing follicle containing a centrally located single prominent radiating vessel and concentric rings of mantle zone lymphocytes, characteristic of Castleman's lymphadenopathy
DISCUSSION
Castleman et al.[
Making a clinical diagnosis of Castleman's lymphadenopathy is difficult as a result of the disease's rarity and asymptomatic presentation and because it can often be mistaken for lymphoma.[
Hanzel et al.[
We describe a rare case of Castleman's disease masquerading as a carotid body tumor. Surgeons who are confronted with similar head and neck pathology should pay close attention to the involvement of the carotid vessel wall, as highlighted in our case. Hints of a different pathology were apparent; however, it was only after a pathological examination that we made the diagnosis of Castleman's disease.
ACKNOWLEDGMENTS
We would like to thank Paul H. Dressel BFA for the preparation of the illustrations and Debra J. Zimmer for editorial assistance.
Dr. Siddiqui declares the following financial relationships (all outside the submitted work): research grants–National Institutes of Health (co-investigator: NINDS 1R01NS064592-01A1 and NIBIB 5 RO1 EB002873-07), University at Buffalo (Research Development Award); financial interests–Hotspur, Intratech Medical, StimSox, Valor Medical, Blockade Medical, Lazarus Effect; consultant–Codman and Shurtleff, Inc., Concentric Medical, Covidien Vascular Therapies, GuidePoint Global Consulting, Penumbra, Stryker Neurovascular, Pulsar Vascular; speakers’ bureaus–Codman and Shurtleff, Genentech; National Steering Committees for Penumbra 3D Separator Trial, Covidien SWIFT PRIME Trial; advisory board–Codman and Shurtleff, Covidien Vascular Therapies; honoraria–Abbott Vascular and Codman and Shurtleff, Inc. for training other neurointerventionists in carotid stenting and for training physicians in endovascular stenting for aneurysms.
References
1. Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956. 9: 822-30
2. Chaloupka JC, Castillo M, Hudgins P. Castleman disease in the neck: Atypical appearance on CT. AJR Am J Roentgenol. 1990. 154: 1051-2
3. Chen CC, Jiang RS, Chou G, Wang CP. Castleman's disease of the neck. J Chin Med Assoc. 2007. 70: 556-8
4. Cronin DM, Warnke RA. Castleman disease: An update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009. 16: 236-46
5. Davidovic LB, Djukic VB, Vasic DM, Sindjelic RP, Duvnjak SN. Diagnosis and treatment of carotid body paraganglioma: 21 years of experience at a clinical center of Serbia. World J Surg Oncol. 2005. 3: 10-
6. Flórez J, García-Pardo G, Auguet T, Sirvent JJ, Bel M, Richart C. Pelvic mass in 46-year old man. Postgrad Med J. 1997. 73: 371-3
7. Hanzel P, Hanzel CV, Hanzel SI, Szepe P, Zelenak K, Hajtman A. Castleman's disease mimicking carotid body tumor. Acta Med Martiniana. 2012. 12: 39-44
8. Manzoor T, Ahmed B, Najam A, Ayub Z. Carotid body paraganglioma. J Coll Physicians Surg Pak. 2009. 19: 523-5
9. Olscamp G, Weisbrod G, Sanders D, Delarue N, Mustard R. Castleman disease: Unusual manifestations of an unusual disorder. Radiology. 1980. 135: 43-8