- Department of Human Neurosciences, Division of Neurosurgery, Policlinico Umberto I University Hospital, Sapienza University of Rome, Rome, Italy
- Department of Neurosurgery, Institute of Neurosurgery, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy
- Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom
- Department of Neurosurgery, Fabrizio Spaziani Hospital, Frosinone, Italy
- Department of DAFR, Pathological Anatomy, Fabrizio Spaziani Hospital, Frosinone, Italy
- Department of Anatomy, Histology, Forensic Medicine and Orthopedics, Policlinico Umberto I Sapienza University of Rome, Rome, Lazio, Italy
Correspondence Address:
Placido Bruzzaniti, Department of Human Neurosciences, Division of Neurosurgery, Policlinico Umberto I University Hospital, Sapienza University of Rome, Rome, Italy.
DOI:10.25259/SNI_539_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Placido Bruzzaniti1, Giovanni Pennisi2, Pierfrancesco Lapolla3, Pietro Familiari1, Vincenza Maiola4, Claudia Quintiliani5, Pierluigi Alò5, Michela Relucenti6, Biagia La Pira4, Giancarlo D’Andrea4. Cerebellopontine angle pilocytic astrocytoma in adults: A systematic review. 04-Oct-2024;15:363
How to cite this URL: Placido Bruzzaniti1, Giovanni Pennisi2, Pierfrancesco Lapolla3, Pietro Familiari1, Vincenza Maiola4, Claudia Quintiliani5, Pierluigi Alò5, Michela Relucenti6, Biagia La Pira4, Giancarlo D’Andrea4. Cerebellopontine angle pilocytic astrocytoma in adults: A systematic review. 04-Oct-2024;15:363. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13129
Abstract
Background: In adults, the cerebellopontine angle (CPA) pilocytic astrocytoma (PA) is very rare. This tumor has radiological features similar to those of a vestibular schwannoma in the few cases reported in the literature.
Methods: In this study, we conducted a systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol and scrutinized all original studies pertaining to pontocerebellar angle PA in adult patients. We conducted an analysis of the clinical, radiological, and molecular components of all eligible articles. We have also reported a case involving a 67-year-old male individual in whom the PA exhibited radiological characteristics similar to an epidermoid cyst.
Results: After the screening phase, we found four cases of PA of the pontocerebellar angle. Three cases were identified that resembled vestibular schwannoma; however, in our case, the tumor resembled an epidermoid cyst. These uncommon tumors exhibit distinctive histological patterns and molecular characteristics (adenosine triphosphate dependent helicase (ATP- dependent helicase)+, Isocitrate dehydrogenase 1−), rendering them a potential differential diagnosis for glioblastoma (GBM).
Conclusion: The CPA PA has rarely been found in adult patients and should be considered in the differential diagnosis of vestibular schwannoma and epidermoid cysts. In these rare cases, the histological characteristics of PA are significant for the differential diagnosis of GBM.
Keywords: Cerebellopontine angle, Epidermoid cyst, Molecular markers, Pilocytic astrocytoma, Tumor
INTRODUCTION
Pilocytic astrocytomas (PAs) account for 5–6% of all brain tumors and are one of the most common primary brain tumors in children.[
MATERIALS AND METHODS
The protocol of the systematic review presented herein was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Protocols guidelines.[
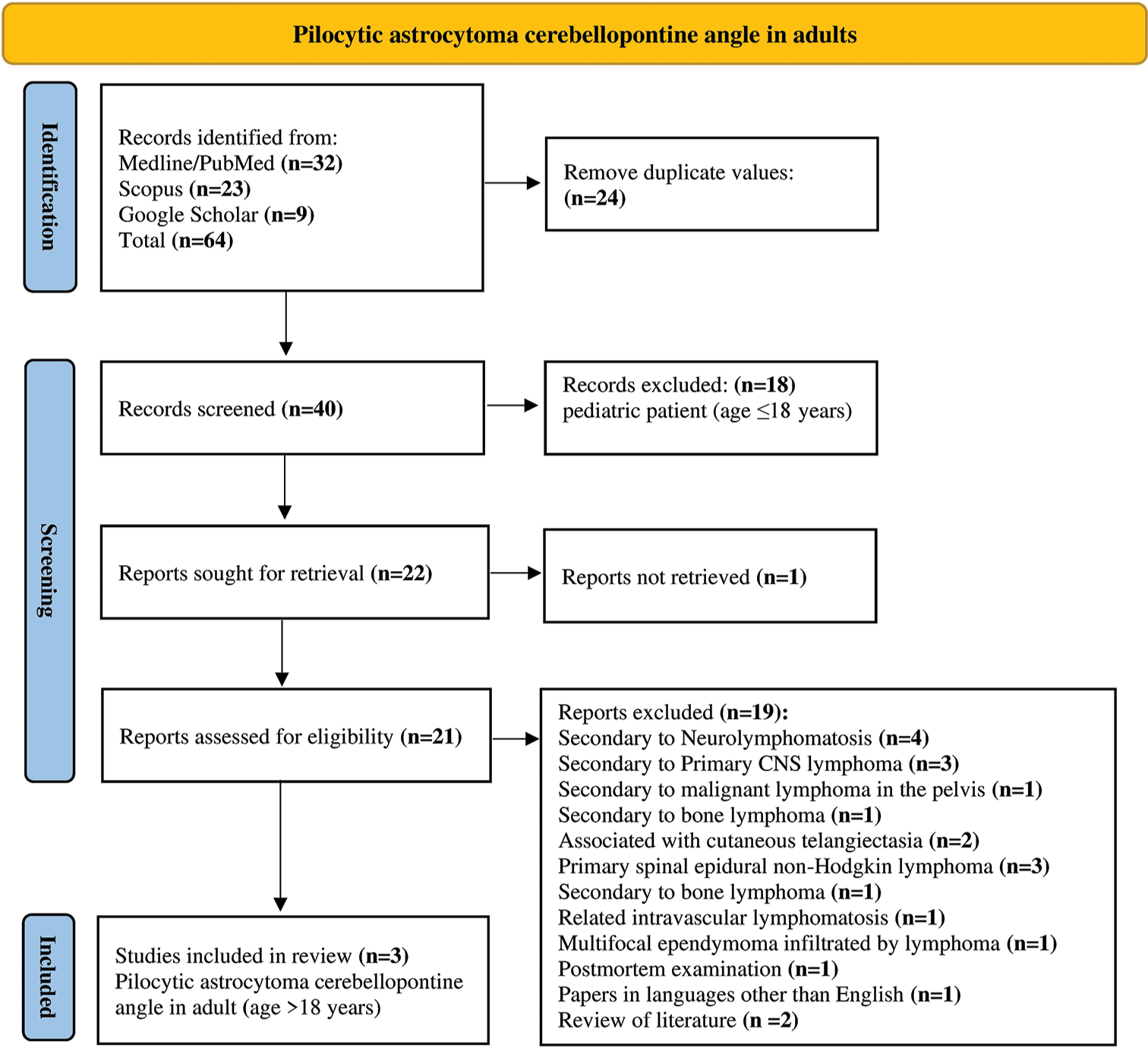
The most recent literature research was conducted in January of 2024, utilizing the databases of Medline/PubMed, Scopus, and Google Scholar. We searched for the following terms: “Astrocytoma,” “Pilocytic Astrocytoma,” “CPA,” or “Cerebellopontine angle,” and “Brain tumor.” Two independent authors, namely, P. B. and G. P., independently assessed the abstracts for their eligibility. Any disagreements were resolved through consensus with a third senior author, namely, G.D. and B.L.P. The publication date was without any restrictions.
There were 64 items identified, 24 of which were duplicates. Eighteen articles have been excluded as they pertain to pediatric patients (age ≤18), and one item was not found. We excluded 19 reports for the following reasons: Secondary to neurolymphomatosis (n = 4); secondary to primary central nervous system (CNS) lymphoma (n = 3); secondary to malignant lymphoma in the pelvis (n = 1); secondary to bone lymphoma (n = 1); associated with cutaneous telangiectasia (n = 2); primary spinal epidural non-Hodgkin lymphoma (n = 3); secondary to bone lymphoma (n = 1); related intravascular lymphomatous (n = 1); multifocal ependymoma infiltrated by lymphoma (n = 1); postmortem examination (n = 1); papers in languages other than English (n = 1); and review of literature (n = 2). Nevertheless, we excluded studies published in languages other than English, pediatric cases (Age ≤18 years), review studies, and meta-analyses. A systematic abstract screening of the references (forward search) was performed to identify additional records. Results obtained were summarized using the PRISMA 2020 flow diagram for new systematic reviews, which included searches of databases and registers only [
Our findings reported in
The surgery procedure was performed with an intraoperative microscope, neuronavigation, and electrophysiological neuromonitoring (Somatosensory evoked potentials (SEP), motor evoked potential (MEP), electroencephalogram (EEG) free run, facial, and acoustic monitoring). The histological and molecular examinations were performed, and sections of hematoxylin-eosin stained sections and immunohistochemistry sections were obtained from the paraffin-embedded and formalin-fixed specimen. It was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of the Department of Neurosurgery at Fabrizio Spaziani Hospital, Frosinone, Italy. For scientific purposes, written informed consent was obtained from the patient.
RESULTS
The literature search resulted in a total of 64 results. After our systematic review reported in
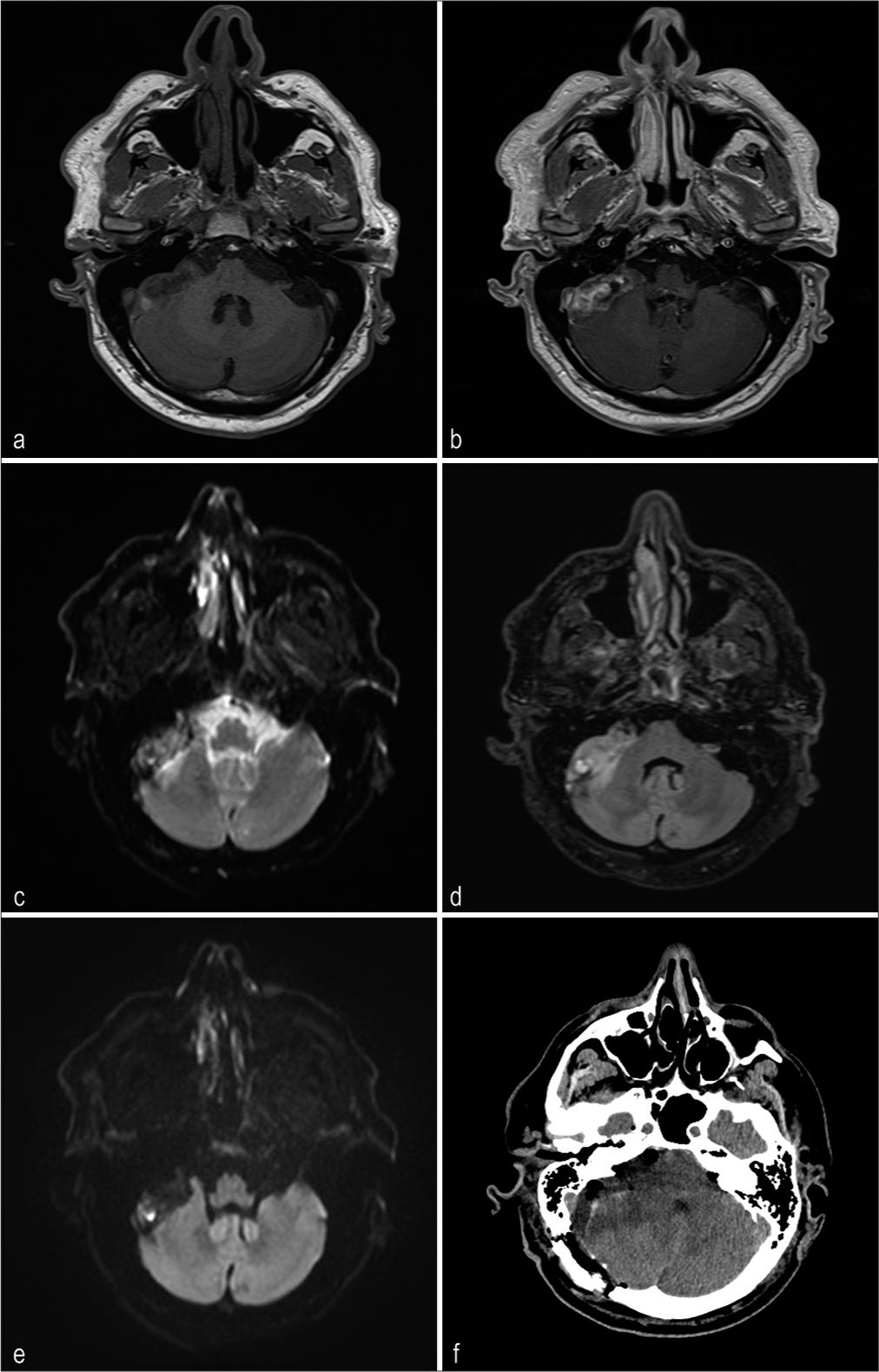
A Caucasian man, aged 67, presented with gait disorders, including ataxic gait, episodes of disorientation, positive Romberg sign, and short-term memory deficits, for approximately 12 months. On magnetic resonance imaging (MRI) of the right cerebellar pons, we observed a tumor fusiform morphology with a maximum dimension of 4.5 × 1.7 cm. The lesion was characterized by hyperintensity both on T2-weighted and fluid-attenuated inversion recovery, accompanied by edema of the adjacent cerebellar tissue. The gadolinium-enhanced T1-weighted sequence revealed an irregular distribution and a predominant concentration at the periphery. Based on the radiological features depicted in
Figure 2:
Magnetic resonance imaging (MRI) of pilocytic astrocytoma of cerebellopontine angle right in a 67-year-old man. (a) The T1-weighted axial MRI demonstrates a lesion of 4.5 × 1.7 cm maximum diameter, exhibiting a hypointense signal. (b) Gadolinium-enhanced T1-weighted axial MRI shows an extra-axial lesion with outfit and not homogeneous signal enhancement. This is mainly noticeable at the periphery of the lesion. (c) In the T2-weighted axial MRI, the lesion exhibits signal hyperintensities and appears to have an interface with nerve tissue. (d) In the T2 – fluid-attenuated inversion recovery-weighted axial MRI, the lesion shows signal hypertension and edema in the cerebellar parenchyma. (e) Diffusion-weighted imaging indicates a restricted diffusion of the lesion. (f) A postoperatively computed tomography scan revealed the presence of air bubbles at the lesion site and moderate edema of the cerebellar parenchyma.
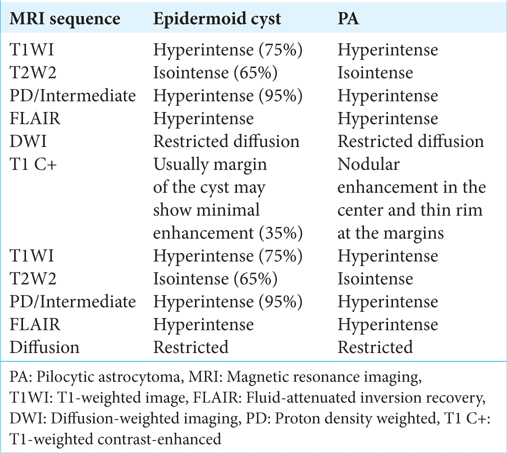
In the present case, MRI revealed that there was a mass in the CPA with characteristics that suggest an epidermoid cyst. The lesion was hypointense on T1-weighted images and hyperintense on T2-weighted images, with no significant enhancement after gadolinium administration. Diffusion-weighted imaging showed a weak restricted diffusion pattern of an epidermoid cyst, as reported in
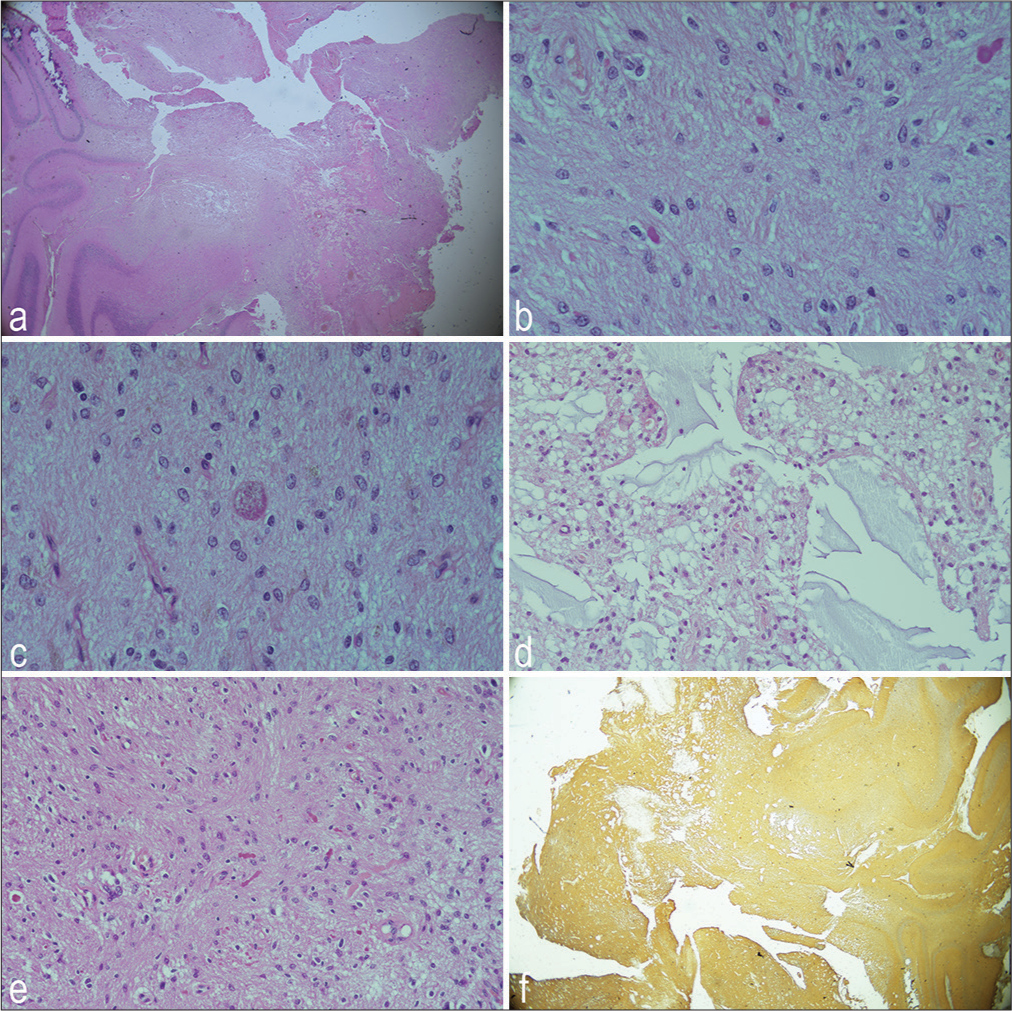
The patient underwent a retrosigmoid craniotomy approach to remove the tumor. An intraoperative neurophysiological evaluation was performed. On examination of the microscope images, it was observed that the tumor originated from the lower cranial nerve (IX–XI) and that a distinct cleavage plane existed between the cerebellum and brain stem. The tumor, which had an irregular margin and was brown-red, occupied the right lateral cerebellomedullary cistern and developed into the cerebellopontine cistern. It had an irregular margin and was brown-red. The condition caused a dislocation of the cochleovestibular facial bundle and involved the cranial nerve VII. Histology was critical in determining whether this tumor was really a primary CPA glial tumor. Histological examination revealed features of PA such as a biphasic growth pattern, an alternation between compact areas with microcystic areas, low proliferation activity, and p53 negative. As shown in
Figure 3:
Histological examination of pilocytic astrocytoma of cerebellopontine angle right in a 67-year-old man. (a) HaematoxilinEosin (H&E) ×10 biphasic growth pattern characterized by compact areas alternating with microcystic areas, (b) H&E ×20 globuli ialini, (c) H&E ×40 granular body, (d) H&E ×20 microcystic areas, (e) H&E Rosenthal fibers, and (f) positive expression of the glial fibrillary acidic protein.
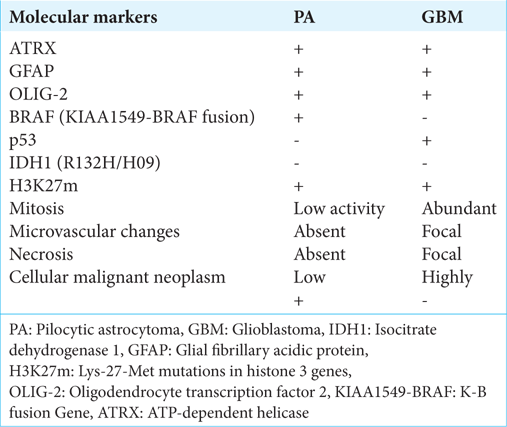
Notwithstanding, the GBM and PA exhibit significant distinctions at the molecular level, which are the root cause of their distinct biological behaviors and clinical outcomes. In our case, the differential diagnosis with GBM proved to be challenging due to the overlapping molecular characteristics, as illustrated in
During the immediate postoperative period, the patient did not experience new-onset neurological deficits, except for a Grade II deficit of the VII ipsilateral cranial nerve second House Brackmann Facial Nerve Grading System. However, he exhibited psychomotor agitation and disorientation in both space and time. This has been improving gradually. During the 3rd day after the surgery, the patient experienced cardiac atrial flutter, accompanied by a high ventricular response, resulting in an irreversible coma. The patient underwent treatment for hydrocephalus through the use of an external ventricular shunt. Heart failure and cardiac arrhythmia can cause an embolic ischemic stroke of the brain stem. The patient passes away within a few weeks. The patient had no previous history of cardiac arrhythmias or other known cardiac disorders, except for arterial hypertension, which was adequately compensated with ramipril therapy.
DISCUSSION
PA is a rare entity in adults, but it is one of the most common tumors in children. Moreover, the supratentorial site is the most common localization, and the pontocerebellar angle is a rare location.[
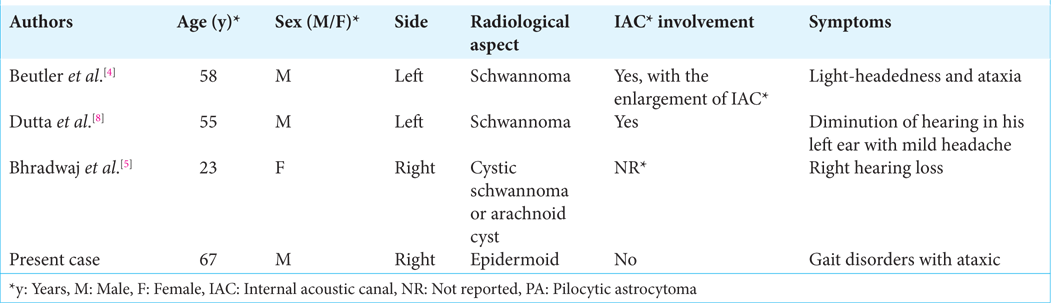
As this condition is extremely rare, there is limited data on the follow-up and outcomes of these patients. Bhradwaj et al. reported a 3-month follow-up showing an improvement in preoperative symptomatic.[
All the papers included in this systematic review were considered suspicious for radiological evidence of schwannoma lesions in CPA. We have reported the first case of PA of CPA with radiological mimicking of an epidermoid cyst.
These authors have further reported that tumors originate from the VIII cranial nerve.[
Additionally, during the intraoperative examination, it was observed that a CPA tumor had a cleavage plane between the brain stem and cerebellum. These features were essential in determining that this tumor was a primary CPA glial tumor, and they emphasize the extremely rare conditions we analyze in this paper.[
Posterior cranial fossa GBM is rare in adults, accounting for <1% of all GBM cases.[
In our literature review , we found that four papers reported the GBM of CPA. A gliosarcoma was identified within one of the tumors. To the best of our knowledge, there have been only four reported cases that have been localized within the CPA. This case presents a challenge due to the involvement of the IAC and the radiological aspect of other tumors of the CPA, which strongly suggests vestibular schwannoma.[
GBM is characterized by a complex array of genetic alterations that contribute to its aggressive nature.[
In contrast, PA typically exhibits a simpler genetic landscape, primarily involving alterations in the BRAF gene. The most common alteration is the KIAA1549-BRAF fusion, which results from a duplication event on chromosome 7 and leads to constitutive activation of the BRAF kinase.[
In contrast to GBM, PA typically does not involve modifications in the p53 or RB pathways, indicating its more benign nature. PA has a less aggressive molecular profile and a slower growth rate than GBM, which is highly malignant and invasive. These molecular distinctions are critical in determining and shaping the clinical management and prognostic outcomes for patients with these tumors.
In our case, the morphological and immunohistochemical features of the lesion are characterized by a positive ATP-dependent helicase (ATRX), a negative Isocitrate dehydrogenase (IDH)1/IDH2, and restricted mitotic and proliferative activity. From a histological point of view, the differential diagnosis was made with GBM[
The role of radiological imaging in the diagnosis and management of CPA tumors is crucial. MRI is the preferred imaging technique, providing comprehensive details on the tumor’s location, size, and association with adjacent structures.
To summarize, the diagnosis of PA of the CPA is exceedingly uncommon among adults. To accurately diagnose this condition, a comprehensive approach is required, including a thorough radiological assessment, intraoperative findings, and a detailed histopathological examination. The unique molecular profile of PA, which is different from GBM, is an essential guide for differential diagnosis and clinical management. This case emphasizes the importance of considering PA in the differential diagnosis of CPA tumors, even in older adults, and emphasizes the necessity of meticulous evaluation to avoid misdiagnosis and ensure suitable treatment.
Limitations
In this study, we present a systematic review of the English literature regarding three cases of PA of CPA and a new case we found in our clinical practice. Few cases possess statistical significance and can only provide indicative and not definitive information. Furthermore, the reported cases are not presented uniformly. Given the limited number of cases, only observational and retrospective studies are feasible.
CONCLUSION
This systematic review demonstrates that PA of the CPA is an uncommon occurrence among adult patients. It is important to consider this factor when considering the differential diagnosis of vestibular schwannoma, epidermoidal cyst, and GBM. We have identified the initial instance of a PA at the CPA, which resembles an epidermoid cyst, in a 67-year-old male. It is possible to distinguish between PA and GBM in the CPA by examining the tumor’s morphological characteristics, including the presence of cellular malignancy, mitotic activity, microvascular changes, and necrosis. Our findings highlight the importance of a thorough radiological and histopathological evaluation in preventing misdiagnosis and ensuring appropriate treatment for these uncommon tumors.
Author’s contributions
P.B., G.P.: Conceptualization. G.D.A., B.L.P.: methodology; P.B., G.P.: Software; G.D.A., B.L.P.: Validation; P.B., P.L.: Formal analysis; C.Q., P.A.: Investigation; P.L., P.F., V.M.: Resources; P.B., G.P.: Data curation; P.B., G.P., P.L.: Writing – original draft preparation; G.D.A., B.L.P., and P.F.: Writing review and editing; V.M.: Visualization; G.D.A., P.F., and P.B.: Supervision; G.D.A., B.L.P. and P.F.: Project administration. All authors have read and agreed to the published version of the manuscript.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Ospedale F. Spaziani (I2022-012816).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
Prof. MD Giovanni Condemi.
References
1. Adams H, Chaichana KL, Avendaño J, Liu B, Raza SM, Quiñones-Hinojosa A. Adult cerebellar glioblastoma: Understanding survival and prognostic factors using a population-based database from 1973 to 2009. World Neurosurg. 2013. 80: e237-43
2. Adib SD, Schuhmann MU, Hempel JM, Bornemann A, Zamora RE, Tatagiba M. Surgical management of primary and secondary pilocytic astrocytoma of the cerebellopontine angle (in adults and children) and review of the literature. Neurosurg Rev. 2021. 44: 1083-91
3. Aibaidula A, Chan AK, Shi Z, Li Y, Zhang R, Yang R. Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol. 2017. 19: 1327-37
4. Beutler AS, Hsiang JK, Moorhouse DF, Hansen LA, Alksne JF. Pilocytic astrocytoma presenting as an extra-axial tumor in the cerebellopontine angle: Case report. Neurosurgery. 1995. 37: 125-8
5. Bhradwaj P, Pandey S, Kumar P, Gupta LN, Bharadwaj M. Pilocytic astrocytoma of the cerebellopontine angle: A rare case. Egypt J Neurosurg. 2022. 37: 27
6. Burkhard C, Di Patre PL, Schüler D, Schüler G, Yaşargil MG, Yonekawa Y. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003. 98: 1170-4
7. D’Alessandris QG, Martini M, Cenci T, DI Bonaventura R, Lauretti L, Stumpo V. Tailored therapy for recurrent glioblastoma: Report of a personalized molecular approach. J Neurosurg Sci. 2023. 67: 103-7
8. Dutta G, Singh D, Singh H, Sachdeva D, Kumar V, Chaturvedi A. Pilocytic astrocytoma of the cerebellopontine angle mimicking vestibular schwannoma: Report of a rare entity. Br J Neurosurg. 2020. 34: 107-9
9. Forshew T, Tatevossian RG, Lawson AR, Ma J, Neale G, Ogunkolade BW. Activation of the ERK/MAPK pathway: A signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009. 218: 172-81
10. Francesco F, Maurizio I, Stefano C, Marina S, Ugo S, Massimo S. Trigeminal nerve root entry zone pilocytic astrocytoma in an adult: A rare case of an extraparenchymal tumor. J Neurooncol. 2010. 97: 285-90
11. Gopalakrishnan CV, Dhakoji A, Nair S, Menon G, Neelima R. A retrospective study of primary cerebellar glioblastoma multiforme in adults. J Clin Neurosci. 2012. 19: 1684-8
12. Hur H, Jung S, Jung TY, Kim IY. Cerebellar glioblastoma multiforme in an adult. J Korean Neurosurg Soc. 2008. 43: 194
13. Jones DT, Kocialkowski S, Liu L, Pearson DM, Bäcklund LM, Ichimura K. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008. 68: 8673-7
14. Kasantikul V, Palmer JO, Netsky MG, Glasscock ME, Hays JW. Glioma of the acoustic nerve. Arch Otolaryngol. 1980. 106: 456-9
15. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
16. Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007. 131: 397-406
17. Mirone G, Schiabello L, Chibbaro S, Bouazza S, George B. Pediatric primary pilocytic astrocytoma of the cerebellopontine angle: A case report. Childs Nerv Syst. 2009. 25: 247-51
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
19. Salunke P, Sura S, Tewari MK, Gupta K, Khandelwal NK. An exophytic brain stem glioblastoma in an elderly presenting as a cerebellopontine angle syndrome. Br J Neurosurg. 2012. 26: 96-8
20. Salvati M, Bruzzaniti P, Relucenti M, Nizzola M, Familiari P, Giugliano M. Retrospective and randomized analysis of influence and correlation of clinical and molecular prognostic factors in a mono-operative series of 122 patients with glioblastoma treated with STR or GTR. Brain Sci. 2020. 10: 91
21. Takada Y, Ohno K, Tamaki M, Hirakawa K. Cerebellopontine angle pilocytic astrocytoma mimicking acoustic schwannoma. Neuroradiology. 1999. 41: 949-50
22. Takami H, Prummer CM, Graffeo CS, Peris-Celda M, Giannini C, Driscoll CL. Glioblastoma of the cerebellopontine angle and internal auditory canal mimicking a peripheral nerve sheath tumor: Case report. J Neurosurg. 2019. 131: 1835-9
23. Wu B, Liu W, Zhu H, Feng H, Liu J. Primary glioblastoma of the cerebellopontine angle in adults: Case report. J Neurosurg. 2011. 114: 1288-93
24. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009. 360: 765-73
25. Yang DX, Jing Y, Xu ZM, Yuan F, Liu YL, Wang GS. Primary glioblastoma of cerebellopontine angle in adult mimicking acoustic neuroma. World Neurosurg. 2019. 122: 48-52
26. Zaidi SA, Amanullah , Jafri SK, Sharif S. Glioblastoma multiforme at internal auditory canal. Surg Neurol Int. 2023. 14: 2