- Department of Transfusion Medicine, District General Hospital, Mannar, Sri Lanka.
- Department of Anaesthesia and Intensive Care, District General Hospital, Mannar, Sri Lanka.
- Department of Paediatrics, District General Hospital, Mannar, Sri Lanka.
- Department of Forensic Pathology District General Hospital, Mannar, Sri Lanka.
- Department of Pathology, District General Hospital, Mannar, Sri Lanka.
DOI:10.25259/SNI_248_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pasindu M. Fernando1, B. M. Munasinghe2, M. D. C. J. P. Jayamanne3, K. A. Jayasundara3, W. S. N. W. B. M. A. G. Arambepola2, Selliah Pranavan4, N. D. Ranathunge5. Cerebral cavernous malformation in a child leading to a fatal subarachnoid hemorrhage – “silent but sinister:” A case report and literature review. 07-Jun-2021;12:253
How to cite this URL: Pasindu M. Fernando1, B. M. Munasinghe2, M. D. C. J. P. Jayamanne3, K. A. Jayasundara3, W. S. N. W. B. M. A. G. Arambepola2, Selliah Pranavan4, N. D. Ranathunge5. Cerebral cavernous malformation in a child leading to a fatal subarachnoid hemorrhage – “silent but sinister:” A case report and literature review. 07-Jun-2021;12:253. Available from: https://surgicalneurologyint.com/surgicalint-articles/10874/
Abstract
Background: Cerebral cavernous malformations (CCMs), otherwise known as cavernous hemangiomas/ cavernomas, are a type of vascular malformation. It is the third most common cerebral vascular malformation, histologically characterized by ectatic, fibrous, blood filled “caverns” with thin-walled vasculature without intervening normal brain parenchyma.
Case Description: Herein, we present a case of an original, spontaneous hemorrhage from a sporadic form of CCM without associated gross developmental venous anomaly in an 11-year-old child, which is an extremely rare occurrence, with the special emphasis on the demographic data of the affected population, risk factors associated with hemorrhage, and correlation of histopathological and radiological findings with an in-depth literature review.
Conclusion: The significant majority of the CCM are clinically occult. Hence, the development of risk assessment tools and guidelines for timely neurosurgical intervention poses a greater clinical challenge for medical experts rendering the management of the affected individuals with CCM in an anecdotal situation. Presentation of life-threatening rebleeds and neurological deficits in the diagnosed population albeit uncommon is possibly preventable outcomes.
Keywords: Cavernoma, Cavernous angioma, Cerebral cavernous malformation, Cerebral, Pediatric, Vascular disorders
INTRODUCTION
Cerebral cavernous malformations (CCMs), otherwise known as cavernous hemangiomas/ cavernomas, are a type of vascular malformation. It is the third most common cerebral vascular malformation, histologically characterized by ectatic, fibrous, blood filled “caverns” with thin-walled vasculature without intervening normal brain parenchyma. The significant majority of the CCM are clinically occult. Hence, the development of risk assessment tools and guidelines for timely neurosurgical intervention poses a greater clinical challenge for medical experts rendering the management of the affected individuals with CCM in an anecdotal situation.
Presentation of life-threatening rebleeds and neurological deficits in the diagnosed population albeit uncommon is possibly preventable outcomes. Herein, we present a case of an original, spontaneous subarachnoid hemorrhage from a sporadic form of CCM without associated gross developmental venous anomaly (DVA) in an 11-year-old child which is an extremely rare occurrence with the special emphasis on the demographic data of the affected population, risk factors associated with hemorrhage, and correlation of histopathological and radiological findings with an in-depth literature review.
CASE DESCRIPTION
An 11-year-old child, Asian male, brought to the ETU of our institution when he suddenly collapsed at home. At the time of presentation, he was in asystolic cardiac arrest. Following a prolonged uninterrupted resuscitation, signs of cardiocerebral revascularization were evident with spontaneous breathing and sinus rhythm in ECG. The patient was intubated and transferred to the ICU for post-resuscitation care. Corroborative history taken from the grandmother later revealed a similar past incidence of short-lived syncopal attack with spontaneous recovery about a year ago which was not investigated. She denied any recent history of trauma to head, past events of persistent headaches, seizures, visual symptoms, or behavioral changes. Except for the presumed syncopal event, neither the medical history nor the family history was significant.
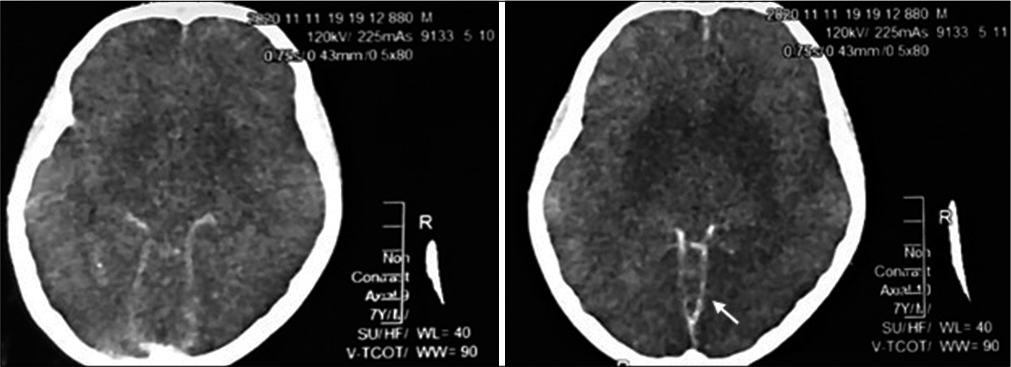
On D1 of ICU care NCCT brain revealed diffuse, superficial subarachnoid hemorrhage (modified Fisher scale Grade 3) [
Electroencephalogram performed on D3 suggested severely reduced cerebral activity. Neuroprotective management was continued with neuroprotective ventilation, maintaining a normocapnia (PaCO2 30–35 mmHg) and normoxia. All the metabolic parameters including serum glucose, electrolytes, lactate, and liver and renal function tests were stable at that moment.
On D7 patient was found to have deteriorating lung functions, setting hypoxia, tachycardia, and elevated temperature with lung signs. Presumptive diagnosis of sepsis with ventilator associated pneumonia was made and after taking appropriate cultures, intravenous antibiotics were escalated. Cerebral diabetes insipidus was diagnosed based on elevated serum Na+ (158–162 mmol/l), polyuria, and suggestive urinary and serum osmolalities (260 mOsmol/kg and 320 mOsmol/kg, respectively).
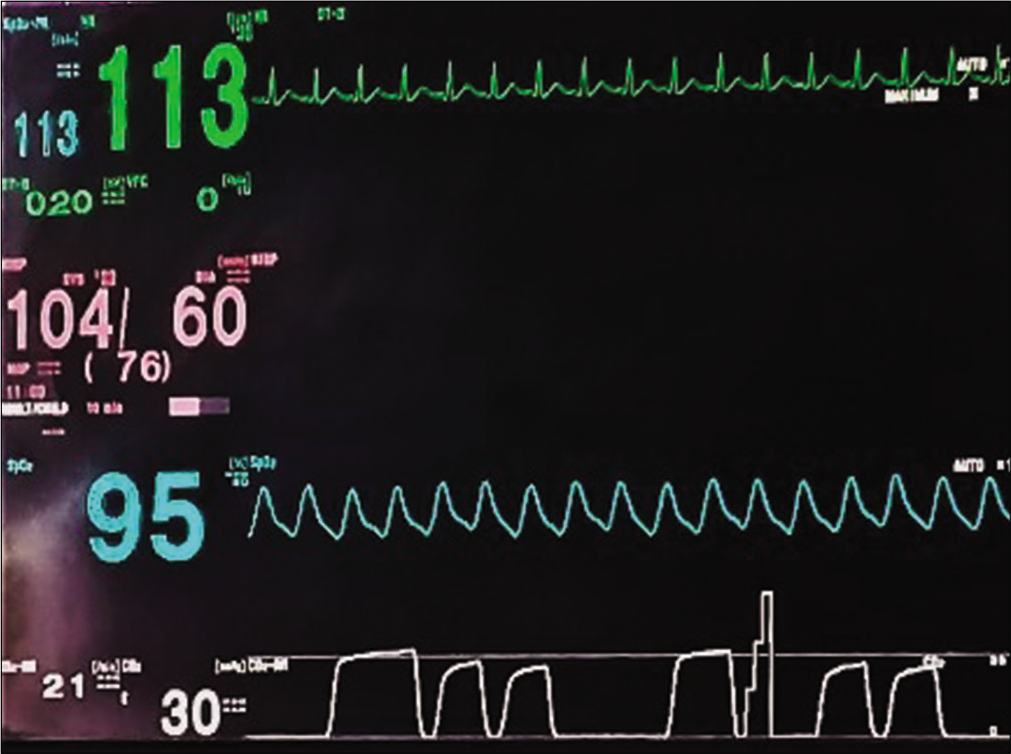
Despite every effort, the patient gradually deteriorated with acute renal failure with metabolic acidosis, persistent hypotension which warranted the use of multiple inotropes, deranged clotting profile with liver failure, and ultimately multiorgan dysfunction. A cluster type respiratory pattern was noticed on capnography [
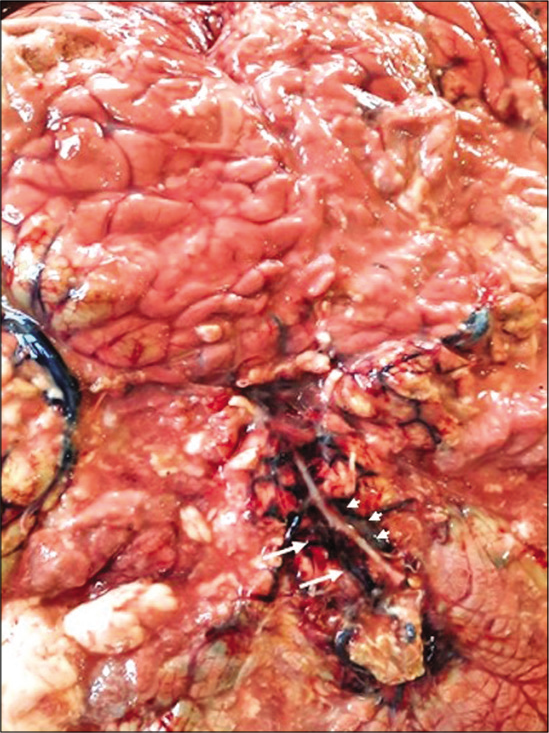
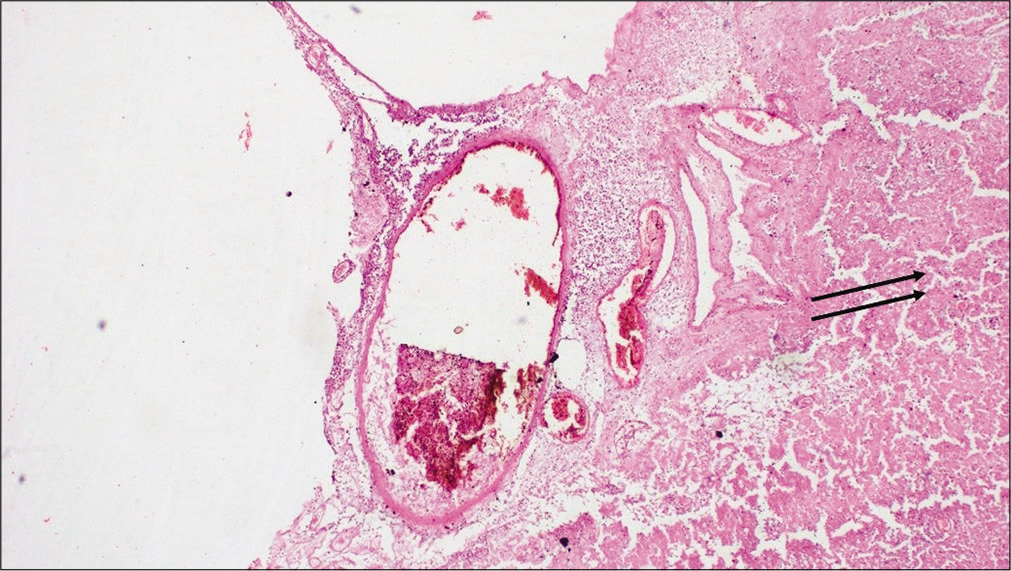
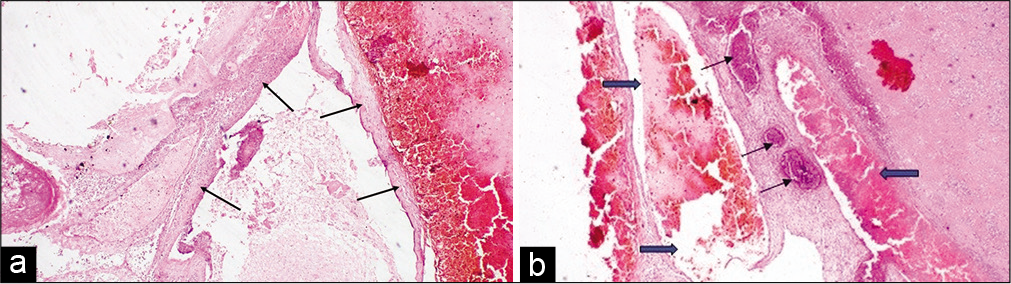
Pathological postmortem revealed extensive SAH with diffused multiple tufts of irregular, dilated vessels in the occipital region of the basilar and interpeduncular cistern area of the cerebrum. Adjacent to the blood clots evidence of pressure effect causing sulci and gyri obliteration with extensive cerebral edema was found [
DISCUSSION
CCMs, also known as cavernous hemangiomas or cavernomas, are a type of vascular malformation found both in inherited and de novo forms.[
CCMs are ectatic, fibrous vessels characterized by “caverns” of blood filled thin-walled vasculature which lack both the elastin and the smooth muscle support in the tunica media [
According to the International Society for the Study of Vascular Anomalies, CCMs are classified as simple nonneoplastic venous malformations.[
The diagnosis of CCM mainly resorts to radiological findings, especially the contrast axial studies. Unless large, these lesions are difficult to see on computed tomography (CT) and were the case in our patient. They do not enhance. If large, they appear as a region of hyperdensity resembling blood products and speckles of calcification. If there has been a recent bleed, then the lesion is more conspicuous and may be surrounded by a mantle of edema,[
Hourihan et al. reviewed a series of SAH in children <20 years and concluded that etiology of SAH is similar to adults but there was a different incidence of the specific pathology producing the bleeding in this series. About 26% of cases were due to bleeding arteriovenous malformations, 52% were due to ruptured aneurysms, and in 19%, no cause was found.[
It is widely accepted that about 0.5% of the general population bear at least a single CCM by the fourth decade of life.[
Results generated by studies vary greatly on the risk of subsequent hemorrhagic presentation of the CCMs, thus, the recommendation for surgical resection is unclear. This poses a great challenge on developing a risk assessment tool for selecting candidates for neurosurgery particularly among the familial CCM group. The size and multiplicity have not been incurred as the risk factors for hemorrhage rate[
CCMs in about 20% of cases (range 2–40%) are concurrently found with DVA in which instance they are named as mixed vascular malformations. Among the risk factors of de novo development of CCMs, nearly half of the sporadic lesions are associated with adjacent DVA. In contrast, the familial CCMs are found in the near absence of DVA in the vicinity.[
CONCLUSION
Although the significant majority of the CCMs may remain clinically silent and the studies conducted in the pediatric population show no exception, in a previously apparently well child who is presenting with a massive hemorrhagic stroke, the possibility of CCM should always be considered. Thorough family history, medical history, and neurological examination are pivotal in the fact finding process. Genetic screening of the first degree kindred of the affected patients if the family history is significant for similar events, sequential radiological follow-up and provident selection of the candidates for neurosurgery who are at risk of rebleeding are of paramount importance in the management of CCM with a prodigious outcome to minimize both the disease affected life years of the patients and health-related cost to the state.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge the Department of Histopathology, Teaching Hospital, Jaffna, Sri Lanka, for the preparation of histopathological specimens.
References
1. Abla AA, Lekovic GP, Turner JD, de Oliveira JG, Porter R, Spetzler RF. Advances in the treatment and outcome of brainstem cavernous malformation surgery: A single-center case series of 300 surgically treated patients. Neurosurgery. 2011. 68: 403-15
2. Bacigaluppi S, Retta SF, Pileggi S, Fontanella M, Goitre L, Tassi L. Genetic and cellular basis of cerebral cavernous malformations: Implications for clinical management. Clin Genet. 2013. 83: 7-14
3. Cisneros O, Rehmani R, de de Jesus KG. Cerebellar cavernous malformation (cavernoma): A case report. Cureus. 2019. 11: e4371
4. Curling OD, Kelly DL, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991. 75: 702
5. Dalyai RT, Ghobrial G, Awad I, Tjoumakaris S, Gonzalez LF, Dumont AS. Management of incidental cavernous malformations: A review. Neurosurg Focus. 2011. 31: E5
6. de Vos IJ, Vreeburg M, Koek GH, van Steensel MA. Review of familial cerebral cavernous malformations and report of seven additional families. Am J Med Genet Part A. 2017. 173: 338-51
7. Fanous AA, Jowdy PK, Lipinski LJ, Balos LL, Li V. Association between trauma and acute hemorrhage of cavernous malformations in children: Report of 3 cases. J Neurosurg Pediatr. 2016. 18: 263-8
8. Fischer A, Zalvide J, Faurobert E, Albiges-Rizo C, TournierLasserve E. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends Mol Med. 2013. 19: 302-8
9. Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 2011. 30: E24
10. Hegde AN, Mohan S, Lim CC. CNS cavernous haemangioma: “popcorn” in the brain and spinal cord. Clin Radiol. 2012. 67: 380-8
11. Hourihan MD, Gates PC, McAllister VL. Subarachnoid hemorrhage in childhood and adolescence. J Neurosurg. 1984. 60: 1163-6
12. Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995. 83: 820-4
13. Kondziolka D, Monaco EA, Lunsford LD. Cavernous malformations and hemorrhage risk. Prog Neurol Surg. 2013. 27: 141-6
14. Kupersmith M, Kalish H, Epstein F, Yu G, Berenstein A, Woo H. Natural history of brainstem cavernous malformations. Neurosurgery. 2001. 48: 47-53
15. Mouchtouris N, Chalouhi N, Chitale A, Starke RM, Tjoumakaris SI, Rosenwasser RH. Management of cerebral cavernous malformations: From diagnosis to treatment. Sci World J. 2015. 2015: 808314
16. Porter P, Willinsky R, Harper W, Wallace M. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. 1997. 87: 190-7
17. Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987. 67: 518-24
18. Uneda A, Yabuno S, Kanda T, Suzuki K, Hirashita K, Yunoki M. Cavernous angioma presenting with subarachnoid hemorrhage which was diffusely distributed in the basal cisterns and mimicked intracranial aneurysm rupture. Surg Neurol Int. 2017. 8: 202
19. Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A. Vascular anomalies classification: Recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015. 136: e203-14
20. Yaghi S, Oomman S, Keyrouz SG. Non-aneurysmal perimesencephalic subarachnoid hemorrhage caused by a cavernous angioma. Neurocrit Care. 2011. 14: 84-5
21. Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP. The natural history of familial cavernous malformations: Results of an ongoing study. J Neurosurg. 1994. 80: 422-32