- Department of Neurological Surgery, FIU Herbert Wertheim College of Medicine, Miami, Florida, United States.
- Department of Radiology, FIU Herbert Wertheim College of Medicine, Miami, Florida, United States.
- Department of Neuro-Oncology, Miami Neuroscience Institute, Miami Cancer Institute, Miami, Florida, United States.
- Department of Neurological Surgery, Miami Neuroscience Institute, Miami Cancer Institute, Miami, Florida, United States.
Correspondence Address:
Douglas J. Chung
Department of Neurological Surgery, Miami Neuroscience Institute, Miami Cancer Institute, Miami, Florida, United States.
DOI:10.25259/SNI_594_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Douglas J. Chung1, Bilal Arif2, Yazmin Odia3, Vitaly Siomin4. Chemotherapy-induced changes in tumor consistency can allow gross total resection of previously unresectable brainstem pilocytic astrocytoma. 13-Jan-2021;12:12
How to cite this URL: Douglas J. Chung1, Bilal Arif2, Yazmin Odia3, Vitaly Siomin4. Chemotherapy-induced changes in tumor consistency can allow gross total resection of previously unresectable brainstem pilocytic astrocytoma. 13-Jan-2021;12:12. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10527
Abstract
Background: Low-grade gliomas (LGG) are described by the World Health Organization as Grades I and II. Among LGGs, the most common primary brain tumor is pilocytic astrocytoma (PA) and carries an excellent prognosis when treated with complete surgical resection. Cases, in which this is not possible, are associated with less favorable outcomes and worse progression-free survival.
Case Description: This report describes a case of a 22-year-old male, who presented with progression of a primary brainstem tumor previously treated with stereotactic radiosurgery and chemotherapy. Patient underwent surgical exploration and was diagnosed with juvenile PA, but debulking was limited by the very dense and fibrous tumor. Complete surgical resection was not possible at this time. Despite efforts to treat with chemotherapy, the patient presented a year later with clinical deterioration and severe neurologic deficits, prompting surgical re-exploration. During the second operation, the tumor was found to have undergone very significant softening in consistency, allowing for gross total resection (GTR)
Conclusion: Aggressive treatment of brainstem LGG should be pursued whenever possible, given its generally favorable prognosis. Repeat microsurgical resection, even with a different approach, might be reasonable and safe. Finally, chemotherapy may be associated with changes in the tumor consistency that can render previously unresectable lesions amenable to successful aggressive resection.
Keywords: Brainstem low-grade glioma, Chemotherapy, Gross total resection, Pilocytic astrocytoma, Tumor consistency
INTRODUCTION
Low-grade gliomas (LGG) have been described according to the World Health Organization (WHO), as Grades I and II.[
PAs are typically well-defined tumors with cystic formation occurring within the tumor or around the tumor with associated solid nodule.[
While PAs are considered to have an excellent prognosis with overall 10-year survival reported over 90%, tumors where complete surgical resection is unable to be carried out have less favorable progression-free survival (PFS) and overall survival.[
However, the repeat microsurgical resection of partially resected tumors that have been treated with chemotherapy resulting in gross total resection (GTR) has not been previously reported. Here, we present a case of a 22-year-old male with an initially unresectable brainstem LGG treated with vincristine and carboplatin, subsequently undergoing changes in consistency allowing for GTR on repeat surgery.
CASE PRESENTATION
History
On March 2015, a 22-year-old right-handed male with a 2 month history of right sided weakness and numbness was diagnosed with a primary brainstem tumor on MRI. He was empirically treated with temozolomide, bevacizumab, and stereotactic radiosurgery (SRS). Six months later, while stable disease was noted on brain MRI, the patient was also treated with an additional hypo-fractionated course (~50Gy in 25 fractions over 2 months) of involved field radiation.
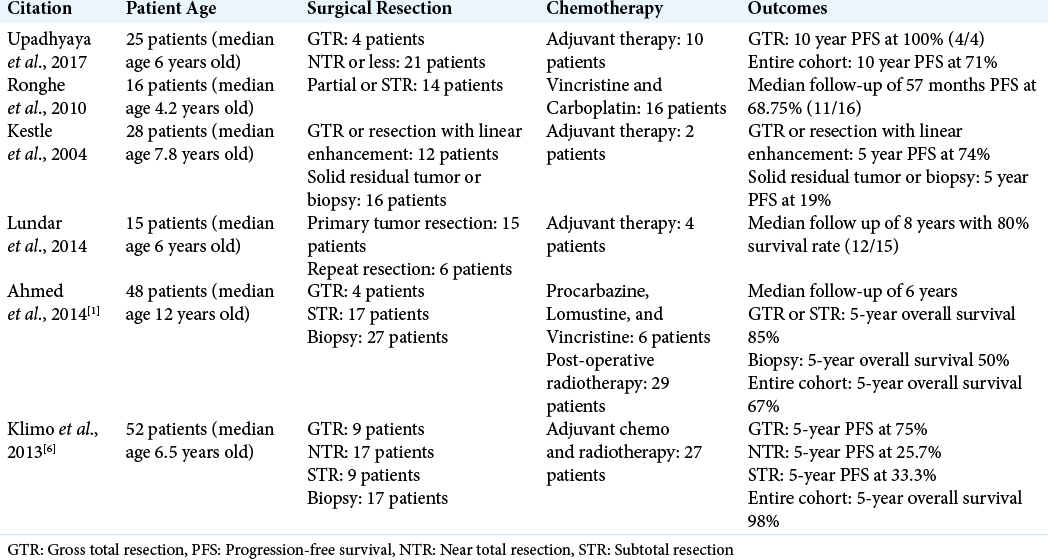
Patient received empiric chemotherapy and radiation therapy for 21 months and follow-up brain MRI on December 2016 revealed a stable, necrotic left midbrain mass lesion measuring 2.5 × 2.1 × 1.8 cm with subacute hemorrhage in the inferior posterior margin [
Nearly 2 years after the initial diagnosis, the patient presented to the clinic with progressively worsening gait instability and left greater than right weakness. Additional symptoms included transient confusion with malaise and dizziness. Brain MRI at this time revealed progression at medial and inferior margins along with interval mild ventriculomegaly consistent with obstructive hydrocephalus [
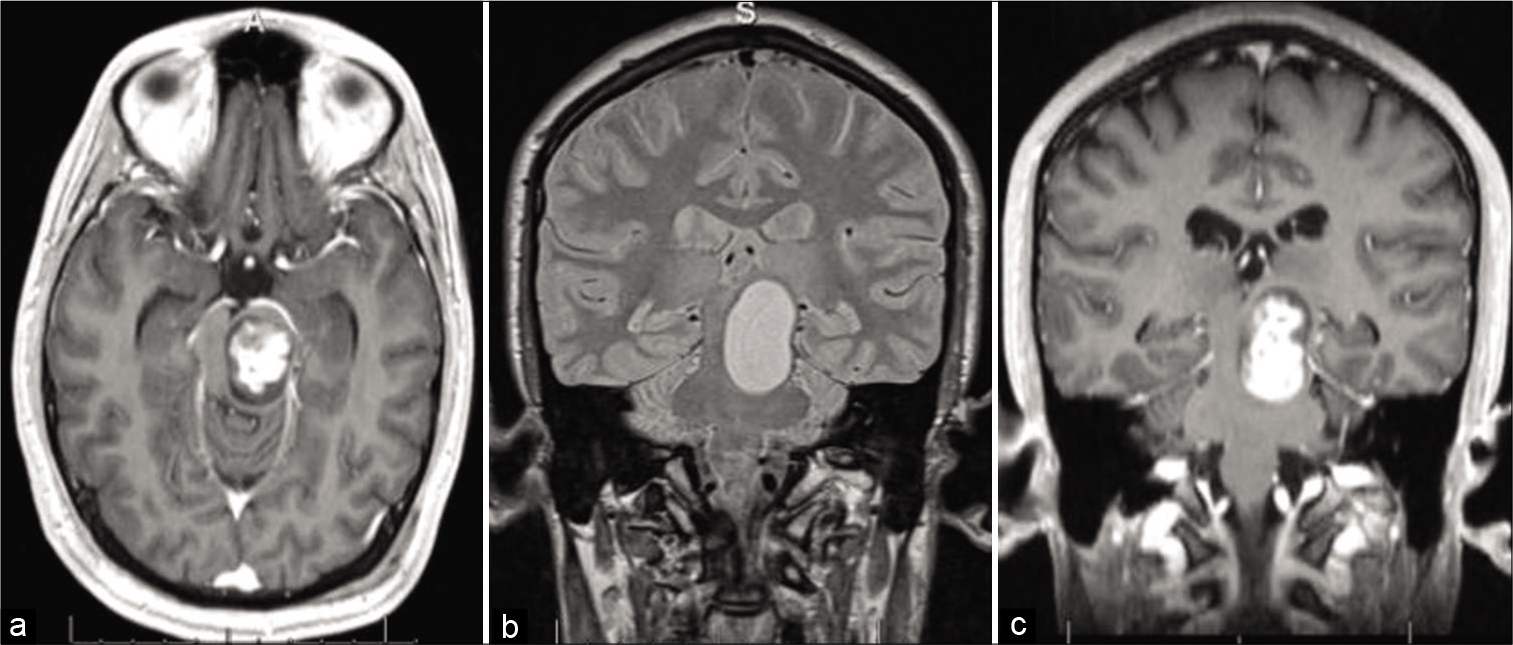
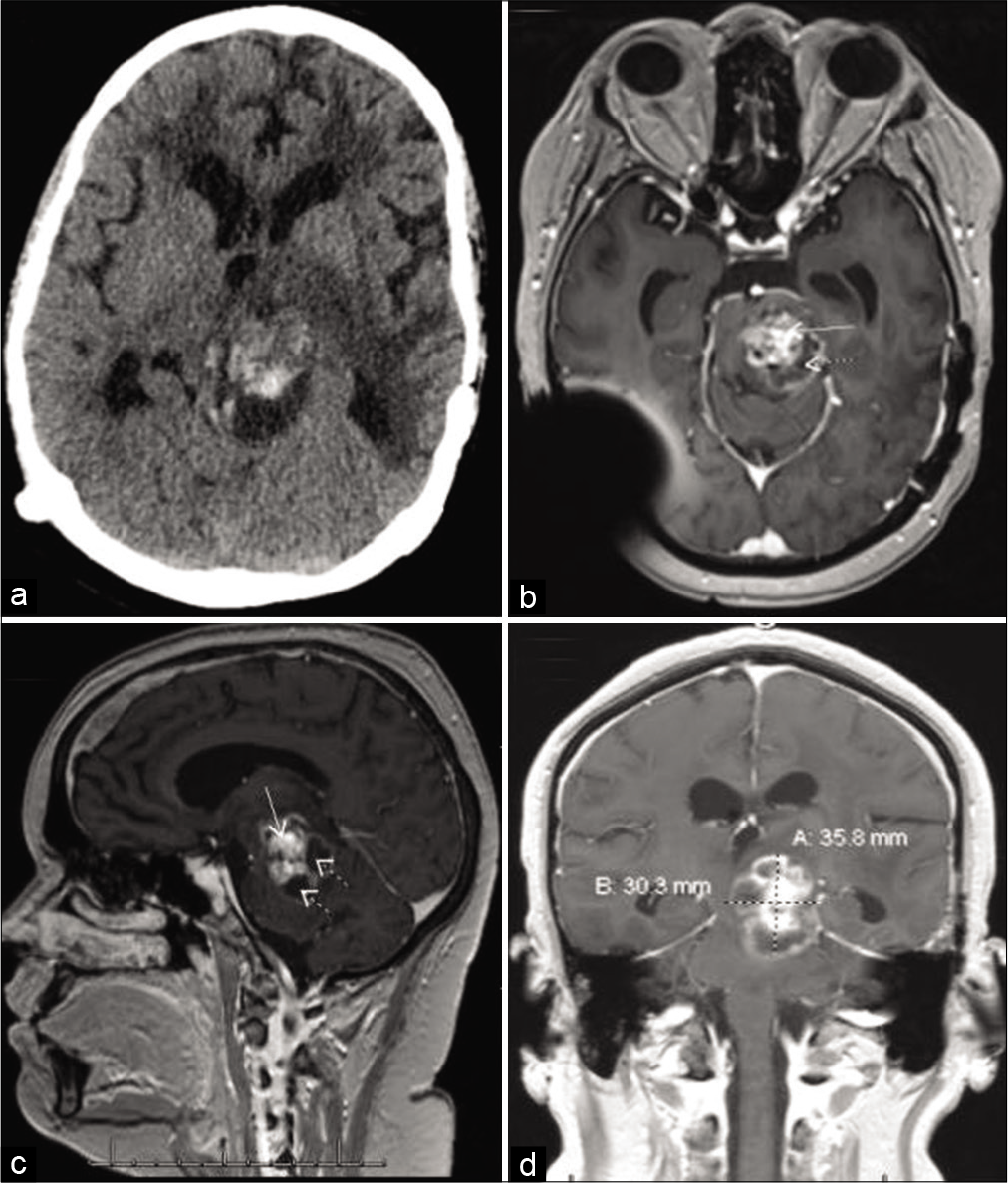
Figure 2:
Two years after stereotactic radiosurgery and first round of chemotherapy: (a) Brain CT showing hemorrhage in the dorsal aspect of the tumor. Brain MRI with contrast: (b) Axial T1 image demonstrating the lesion in the left brainstem and prominent temporal horns (arrows), suggestive of an obstructive hydrocephalus; (c) sagittal T1 image showing ring-enhancing mass.
First operation – VP shunt placement and partial tumor resection/biopsy
The patient underwent an uneventful right occipital ventriculoperitoneal shunt placement with utilization of volumetric image guidance and laparoscopic assistance.
The following day, left posterior temporal-occipital craniotomy was performed with an intention to obtain tissue for diagnosis and remove as much of the brainstem tumor as safely as possible. Volumetric image guidance, microscope, neuro-monitoring, and intraoperative MRI were utilized. The brainstem lesion was approached through the posterior temporal-occipital supratentorial plane with sectioning of the tentorium to widen the exposure. The inferior posterior temporal gyrus was partially resected, allowing mobilization and preservation of the vein of Labbe. The midbrain was entered through the lateral mesencephalic safe entry zone. The tumor itself was very firm and moderately vascular. Usual microsurgical tools, including ultrasonic aspirator, were inefficient.
The tumor’s firm consistency did not allow us to remove much of the lesion. The outer portions of the mass were very fibrous and could not be mobilized. Progress was very slow. The remaining tumor was “hard as a rock” in consistency, and surgical manipulation would move it as a single block, distorting the entire brainstem. Considering the circumstances, it was felt that the safest choice would be to stop further dissection/tumor removal. Intraoperative MRI showed an approximately 40% decrease in tumor volume, but the peripherally enhancing capsule of the tumor remained unchanged, as expected given the consistency of the tumor encountered intraoperatively [
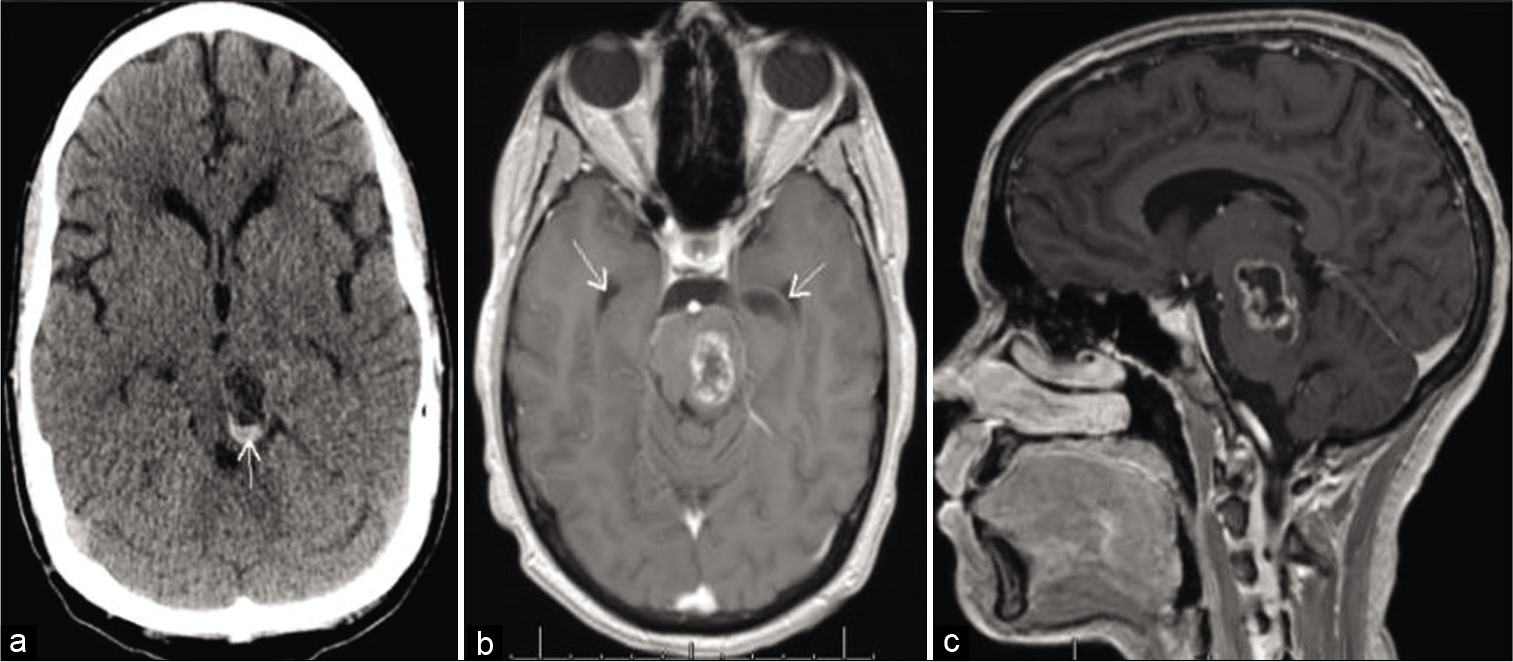
Figure 3:
MRI images with contrast: (a) Immediate preoperative axial T1 views; (b) intraoperative Axial T1 views demonstrate an approximate 40% resection of the lesion (dotted arrow) and expected intraoperative pneumocephalus (solid arrows); (c) immediate preoperative coronal T1 views; (d) intraoperative coronal T1 views.
Pathology and postoperative course
Final pathology confirmed PA, negative for BRAF mutation or rearrangement, and the patient was started on combination therapy with carboplatin and vincristine. Unfortunately, chemotherapy was complicated with breakthrough seizures and brain MRI revealed symptomatic interval progression of the left midbrain tumor. Carboplatin and vincristine were discontinued, and seizures controlled with levetiracetam, gabapentin, and valproic acid. The patient was subsequently started on a 3 day monthly cycle of cisplatin and etoposide.
On May 2018, 14 months after the first operation, the patient presented to the emergency department for worsening headache, double vision, right-sided weakness, and gait ataxia. Clinically, he was noted to have significant right upper extremity weakness to 1–2/5, hypophonia, and rapidly progressing functional decline. Brain CT showed known mass extending from the brainstem to the thalamus, slightly eccentric on the left. A hemorrhagic component within the mass and slight increase in size of the tumor was noted as well [
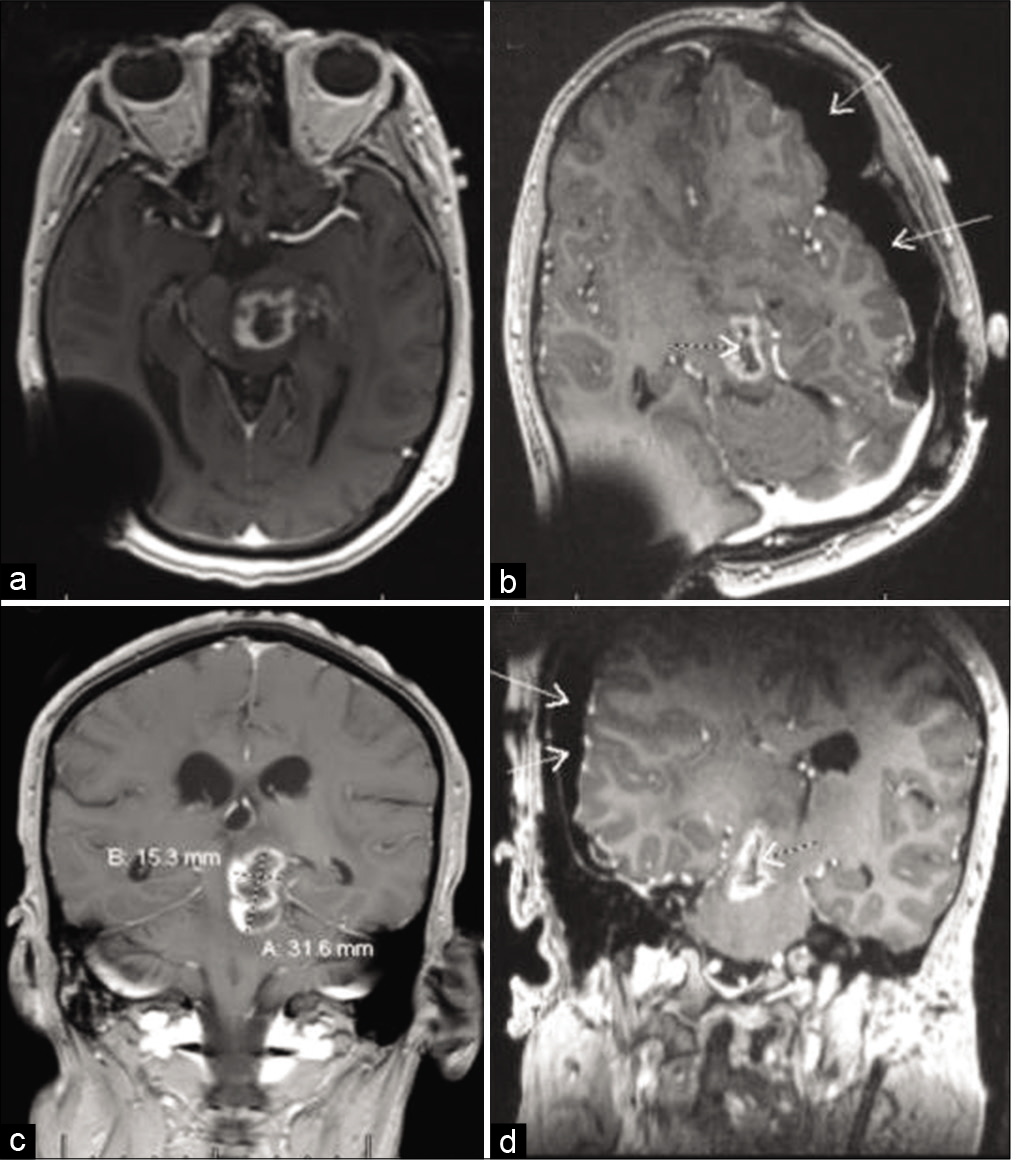
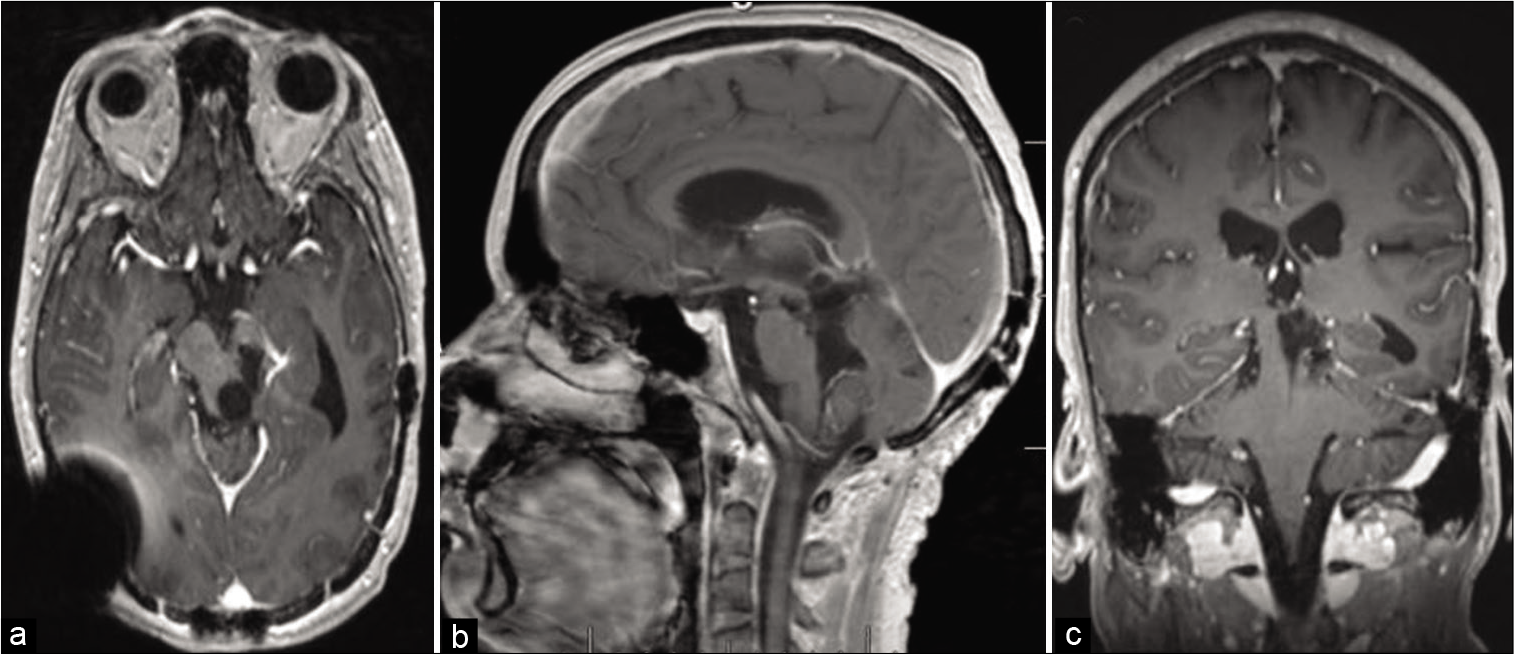
Figure 4:
Four months after partial resection and shunting; (a) non-contrast brain CT shows hemorrhagic component mostly in the posterior aspect of the tumor; (b) axial, (c) sagittal, and (d) coronal T1 MRI views with contrast, showing significant interval tumor enlargement with solid (solid arrows) and cystic (dotted arrows) components. At this point in the patient’s care, it was felt, he did not have many options left. He appeared to have either poorly tolerated, or did not respond to, chemotherapy. After two prior radiation treatments, he was ineligible for further radiation. Therefore, a multi-disciplinary decision was made to repeat neurosurgical intervention in an attempt to decompress the cyst and remove some more tumor, if feasible.
Second operation
The patient underwent suboccipital-torcular craniotomy with utilization of volumetric image guidance, microscope, and neuromonitoring. The lesion was approached through the supracerebellar infratentorial corridor through the infracollicular safe entry zone. Initially, the cystic portion was decompressed and motor oil-like fluid evacuated. The cyst walls collapsed and the brainstem relaxed. The solid portion of the tumor in the middle of the caudal midbrain and upper pons was considerably softer than in the first surgery, but more vascular with areas of hemorrhagic transformation. This tumor was successfully mobilized and grossly resected with what appeared to be normal appearing brainstem underneath.
Postoperative brain MRI demonstrated GTR and marked decompression of the brainstem. Pathology confirmed mainly necrosis and hemorrhage with focal residual PA with little/no proliferation and Ki-67 in MID1 proliferation indices [
Postoperative complications
Five days after surgery, the patient experienced increased somnolence. Head CT revealed new bilateral parieto-occipital subdural and epidural retrocerebellar hemorrhage. The parieto-occipital convexity hemorrhages were likely related to intra- and postoperative brain shift and tearing of the bridging veins. Although supratentorial hematomas were not felt to require surgical intervention, the retrocerebellar hemorrhage was more significant. The patient was subsequently taken up for emergent re-opening of the torcular-suboccipital craniotomy for evacuation of the epidural hematoma. Postoperative CT revealed successful evacuation of the extra-axial hematoma.
Three months after surgery, follow-up brain MRI demonstrated evolving postoperative changes with no residual/recurrent tumor.
Twelve months after surgery, the patient’s dysarthria improved. Motor exam revealed persistent right hemiparesis (improved to 3–4/5), arm weaker than the leg. Patient was now able to stand and walk short distances with assistance. Chronic steroids have been successfully weaned off, and the patient had a complete reversal of cushingoid appearance.
DISCUSSION
LGG have been described according to the WHO, as Grades I and II.[
The gold standard for approaching pediatric brainstem LGGs is initial treatment with safe maximal surgical resection, in conjunction with chemotherapy and radiation therapy as needed.[
Kestle et al.’s study investigated 28 cases of pediatric brainstem PA, of which 25 were treated with neurosurgical resection.[
While GTR of brainstem PA clearly leads to favorable outcomes, adjuvant or neoadjuvant radiation therapy may also be used in the management of these tumors. Gagliardi et al. investigated the use of radiotherapy for LGGs in 39 patients.[
The patient in this case report was not offered surgery as the first line of treatment. Instead, he was treated with SRS, another hypofractionated course of radiotherapy, and chemotherapy. It is conceivable that the tumor could acquire its very firm consistency as a consequence of these treatments. Hardening of the tumor due to radiation therapy is a well described phenomenon.[
We did not find any reports of repeat surgical interventions on adult patients with brainstem LGG. One study reported 12 pediatric patients with midbrain LGG who were treated with multiple repeat tumor resections due to disease progression with seemingly stable long-term results obtained in at least nine patients.[
CONCLUSION
Aggressive treatment of brainstem LGG should be pursued whenever possible, given its generally favorable prognosis. Repeat microsurgical resection, even with a different approach, might be reasonable and safe. Finally, chemotherapy may be associated with changes in the tumor consistency that can render previously unresectable lesions amenable to successful aggressive resection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahmed KA, Laack NN, Eckel LJ, Orme NM, Wetjen NM. Histologically proven, low-grade brainstem gliomas in children 30-year experience with long-term follow-up at mayo clinic. Am J Clin Oncol. 2014. 37: 51-6
2. Burkhard C, di Patre PL, Schüler D, Schüler G, Yaşargil NG, Yonekawa Y. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003. 98: 1170-4
3. Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015. 129: 775-88
4. Gagliardi F, Bailo M, Spina A, Donofrio CA, Boari N, Franzin A. Gamma knife radiosurgery for low-grade gliomas: Clinical results at long-term follow-up of tumor control and patients quality of life. World Neurosurg. 2017. 101: 540-53
5. Kestle J, Townsend JJ, Brockmeyer DL, Walker M. Juvenile pilocytic astrocytoma of the brainstem in children. J Neurosurg. 2004. 101: 1-6
6. Klimo P, Panandiker AS, Thompson CJ, Boop FA, Qaddoumi I, Gajjar A. Management and outcome of focal low-grade brainstem tumors in pediatric patients: The St. Jude experience. J Neurosurg Pediatr. 2013. 11: 274-81
7. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
8. Lundar T, Due-Tonnessen BJ, Egge A, Scheie D, Brandal P, Stensvold E. Neurosurgical treatment of pediatric low-grade midbrain tumors: A single consecutive institutional series of 15 patients. J Neurosurg Pediatr. 2014. 14: 598-603
9. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018. 20: iv1-86
10. Stubblefield MD. Radiation fibrosis syndrome: Neuromuscular and musculoskeletal complications in cancer survivors. PM R. 2011. 3: 1041-54
11. Upadhyaya SA, Koschmann C, Muraszko K, Venneti S, Garton HJ, Hamstra DA. Brainstem low-grade gliomas in children-excellent outcomes with multimodality therapy. J Child Neurol. 2016. 32: 194-203