- Department of Neurological Surgery, Rush University Medical Center, Chicago, Illinois, USA
- Section of Neurological Surgery, Legacy Emanuel Hospital, Portland, Oregon, USA
Correspondence Address:
David E. Adler
Section of Neurological Surgery, Legacy Emanuel Hospital, Portland, Oregon, USA
DOI:10.4103/sni.sni_304_16
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Josha A. Woodward, David E. Adler. Chiari I malformation with acute neurological deficit after craniocervical trauma: Case report, imaging, and anatomic considerations. 23-Apr-2018;9:88
How to cite this URL: Josha A. Woodward, David E. Adler. Chiari I malformation with acute neurological deficit after craniocervical trauma: Case report, imaging, and anatomic considerations. 23-Apr-2018;9:88. Available from: http://surgicalneurologyint.com/surgicalint-articles/chiari-i-malformation-with-acute-neurological-deficit-after-craniocervical-trauma-case-report-imaging-and-anatomic-considerations/
Abstract
Background:In patients with Chiari I malformation (CMI), the occurrence of acute neurologic deficit after craniocervical trauma is rare. However, the pathologic potential of exacerbating anatomic overcrowding of the posterior fossa has immense clinical consequences and prompt recognition is essential.
Case Description:This case study describes a 41-year-old male who sustained a single blow to the face, fell, and struck the occiput. On admission, neurological examination revealed a profound paraparesis, upper extremity diplegia, a C4 sensory level and apnea that required intubation. On arrival, computerized axial tomography of the head showed a small amount of contrecoup left frontal traumatic subarachnoid hemorrhage. Magnetic resonance imaging (MRI) performed 19 h after admission was negative except for the presence of a CMI. He acutely declined on post injury day 2, prompting emergent decompression of the posterior fossa where anatomic overcrowding was observed. At 19 weeks post injury, his motor function had significantly improved.
Conclusion:The constellation of severe neurologic deficit in patients with CMI after relatively minor craniocervical trauma has been previously described. In our patient, neurologic deficit disproportionate to the mechanism of injury was observed and likely in part attributed to the presence of a Chiari malformation. Unfortunately, MRI has not yet been able to clearly define the underlying pathoanatomy, help understand the mechanism of injury, and delineate when operative intervention is indicated in these patients. Here, we review similar cases from the literature, examine findings on MRI, and evaluate mechanisms of injury following craniocervical trauma in patients with CMI to help clarify these questions.
Keywords: Chiari I malformation, central cord syndrome, craniocervical trauma
INTRODUCTION
Chiari I malformation (CMI) of the adult type is considered rare and its incidence is uncertain.[
It is difficult to diagnose CMI because of these variable signs and nonspecific symptoms. MRI usually confirms the diagnosis. A certain group of patients may present acutely following trauma or in a delayed fashion. In Milhorat's[
Table 1
The type of trauma is presented as head, cervical flexion/extension or a combination thereof. Radiographic imaging modalities were recorded as (CT) computed tomography or (MR) magnetic resonance. X-ray is recorded in the case where it was the only imaging study performed. Time contained in parentheses represents the interval between admission and initial imaging study. Clinical improvement was qualitatively assessed as none, minimal or significant. Surgical intervention was recorded as 1, suboccipital craniectomy or 2, cervical laminectomy
CASE REPORT
History and examination
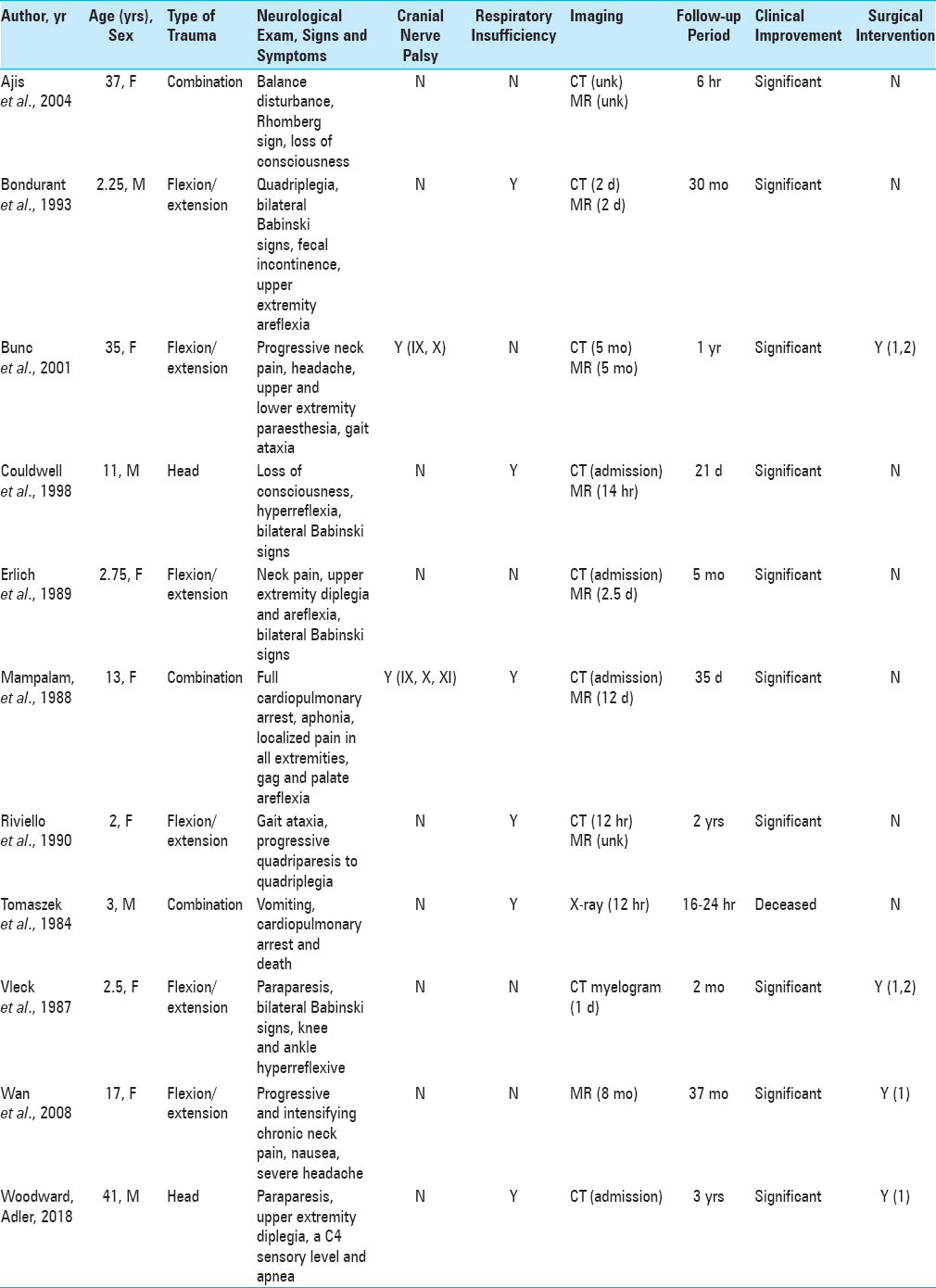
The patient is a 41-year-old male who was sucker punched in the face, fell, and struck his occiput on a curb. A bystander initiated cardiopulmonary resuscitation. Glasgow Coma Score was reported to be 3 when emergency medical service arrived. He was intubated in the field. Blood pressure was 67/47 mm Hg. 0.4 mg of Atropine was given, which increased the blood pressure to 123/94 mm Hg. Upon arrival to the emergency department, the patient was found to be awake, alert, and attentive. Neurological examination showed a C4 sensory level with an upper extremity diplegia. The right lower extremity showed 3/5 strength in all muscle groups. The left lower extremity showed 1–2/5 strength in the plantar and dorsiflexors and absent strength proximally. Computerized axial tomography of the head showed left frontal traumatic subarachnoid hemorrhage. On admission, all other radiographic studies were normal. Sagittal MRI demonstrated 7 mm of tonsillar descent below the foramen magnum, consistent with CMI [
Figure 1
Preoperative. Nineteen hours after admission. Sagittal T2-weighted MR image (TR 3116.7 TE 102.0) of the CMJ shows 7 mm of tonsillar herniation (arrowhead). The subarachnoid space is absent and the CMJ is indented by the dens (arrow). There is no signal change suggesting acute injury. The reference line indicates the anatomical level of the axial image in
Surgery and operative findings
A second preoperative MRI study of the brain was performed the morning of surgery. A cine cerebrospinal fluid (CSF) flow study showed qualitatively decreased flow at the level of the foramen magnum. T2-weighted MRI demonstrated hyperintense signal in the pyramids at and below the foramen magnum and in the dorsal columns of the spinal cord [Figures
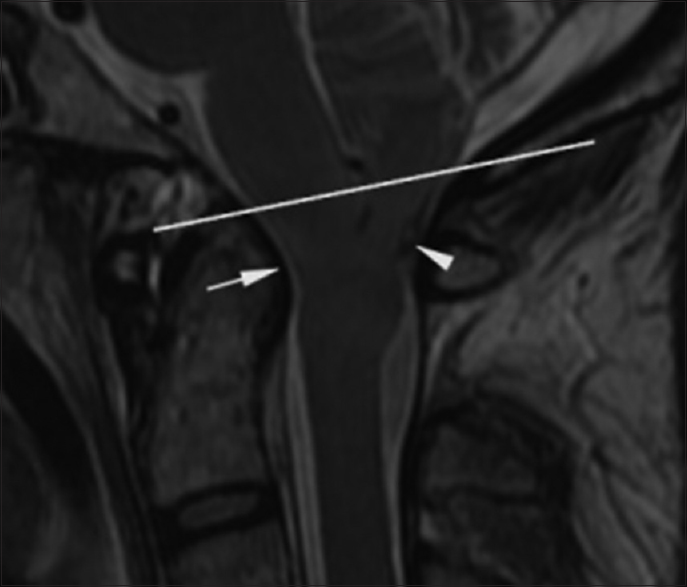
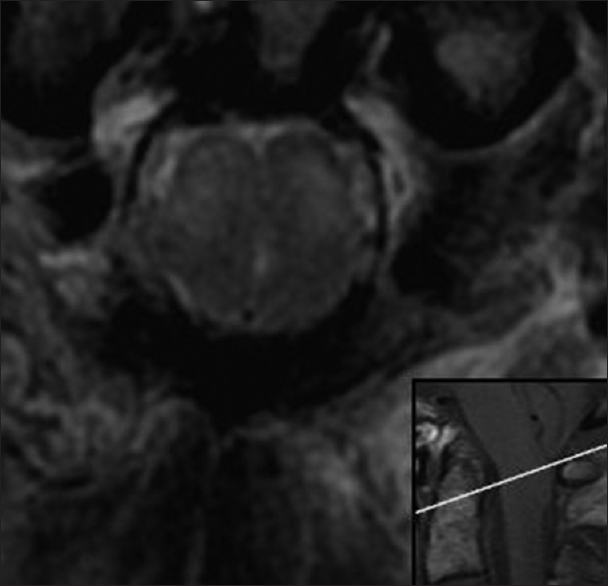
Figure 3
Preoperative. Sixty-one hours post injury. Axial T2-weighted MR image (TR 7200.0 TE 119.6) at the level of the foramen magnum shows hyperintense signal in the medulla in a paramedian location (short arrow) and in the dorsal columns (long arrow). The medulla is compressed by the cerebellar tonsils. The reference line of the inset sagittal image corresponds to the anatomic level of the axial image
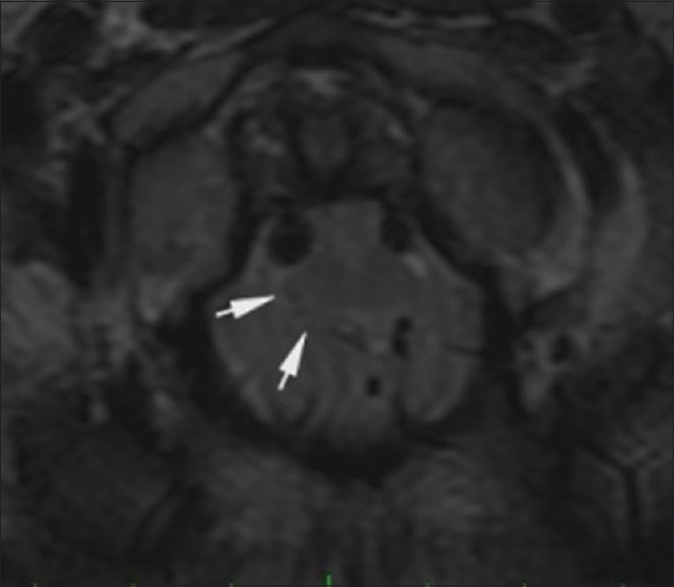
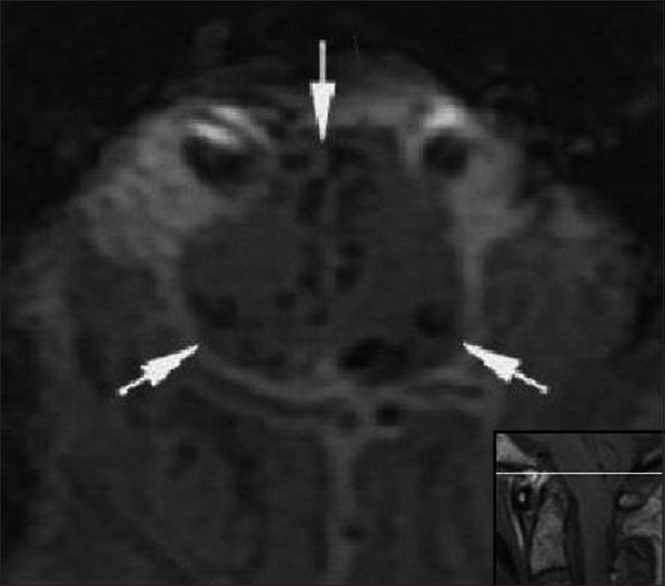
Figure 4
Preoperative. Sixty-one hours post injury. Axial T2-weighted MR image (TR 7200.0 TE 119.6) at the level of the dens shows hyperintense signal in the anterolateral aspect of the rostral cervical spinal cord. The medulla is compressed by the cerebellar tonsils. The reference line of the inset sagittal image corresponds to the anatomic level of the axial image
Postoperative course
On postoperative day 2, right intrinsic hand strength improved to 1/5. Antigravity strength was present in all muscle groups of the right lower extremity. The left lower extremity remained plegic. The C4 sensory level was unchanged. A tracheostomy was performed on postoperative day 7 for persistent apnea. Postoperative cine MRI showed restoration of CSF flow at the level of the foramen magnum. Three weeks post injury, T2-weighted hemorrhagic gradient echo sequence MRI (TR 867, TE 35, and FA 20) showed low-intensity signal in the ventral medulla, bilaterally along the midline and in the dorsal columns. A postoperative pseudomeningocele was identified and aspirated percutaneously without recurrence. Neurological examination remained unchanged by the time of discharge 3 weeks later. Nineteen weeks post injury, the patient showed significant improvement. Antigravity strength was present in all muscle groups. Sensation to pinprick returned to normal in all extremities. The patient ambulated with assistance and was being actively weaned from the ventilator but remained in a rehabilitation institute.
DISCUSSION
Surgical management
The decision to operate on a patient with CMI who becomes symptomatic after CCT is not clear-cut. A review of the literature showed 10 such patients without syringomyelia, hydrocephalus, or other craniospinal abnormality [
The majority of patients in this small series improved both with and without surgery. However, the enhanced risk of neurological deficit after trivial CCT is worth attention and is an important criterion to consider the decision to perform prophylactic decompression in certain patients with CMI. Selected surgical series document rates of improvement in patients with CMI who have not experienced trauma between 80 and 90% and a 10–21% rate of relapse or persistence of symptoms.[
Magnetic resonance imaging
MRI is the best radiographic modality for determining spinal cord and brainstem injury. However, within the first 12 to 24 h, signal change may be difficult to detect or distinguish from artifact, especially at the level of the skull base. In the cases reviewed, T2-weighted MRI demonstrated hyperintense signal in the spinal cord and brainstem. This finding was characterized as edema,[
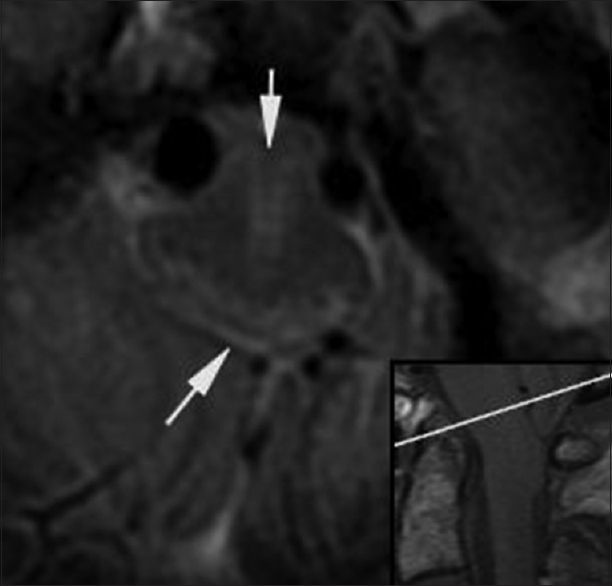
Figure 5
Postoperative. Twenty-one days post injury. Axial T2*-weighted hemorrhagic gradient echo sequence MR image (TR 867.0, TE 35.0 and FA 20) shows hypointense signal in the medulla consistent with the deposition of hemosiderin at the level of the foramen magnum in a paramedian and dorsal location (arrows). The medulla is decompressed with presence of a subarachnoid space. The reference line of the inset sagittal image corresponds to the anatomic level of the axial image
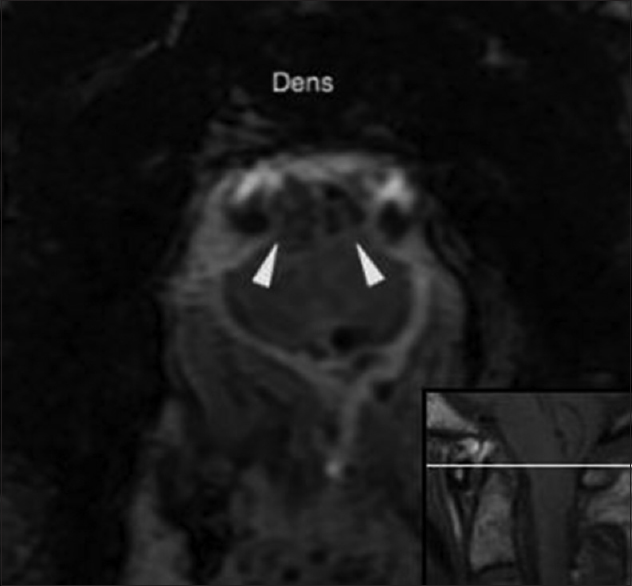
Figure 6
Postoperative. Twenty-one days post injury. Axial T2*-weighted hemorrhagic gradient echo sequence MR image (TR 867.0, TE 35.0 and FA 20) at the level of the dens shows hypointense signal within the ventral cervical spinal cord consistent with the deposition of hemosiderin (arrowheads). The reference line of the inset sagittal image corresponds to the anatomic level of the axial image
Mechanisms of injury
Herniation of the cerebellar tonsils minimizes space below the foramen magnum and increases the risk of injury to the brainstem and spinal cord at the CMJ. In our patient, preoperative axial MRI show asymmetric dorsolateral compression of the brainstem from the cerebellar tonsils. The dens is angulated and protuberant posteriorly and compromises the spinal canal most at this level. There is no evidence of CSF at the level of the CMJ, which is indented ventrally by the dens. The medulla is effaced, shifted anteriorly to the left, and contiguous with the posterior margin of the odontoid process [Figures
In this patient, we suggest that flexion and/or extension of the atlantooccipital junction created a direct and forceful movement of the medulla and brainstem into the posterior aspect of the dens. Here, the spinal canal is made most narrow by the tonsils dorsally and the odontoid process ventrally. Sensory deficit appears to be less common and was not present in any of the cases reviewed. The C4 sensory level in our patient was a function of dorsal column involvement, albeit relatively distant from the posterior margin of the odontoid process and the direct point of trauma. Signal change in the dorsal columns was observed, implicating involvement of the cuneate and gracilis nuclei and fasciculi.
Several other mechanisms for traumatic brainstem and spinal cord injury in patients with CMI have been proposed but none are agreed upon. It is postulated that with blunt head trauma, a transient increase in intracranial pressure can promote further herniation of cerebellar tissue.[
In some of the cases reviewed, motor deficit was a common denominator, suggesting pyramidal tract involvement. In the case presented by Erlich,[
There is no definitive explanation for cruciate paralysis. Those explanations that have been proposed include: (1) Somatotopic organization of the pyramidal decussation;[
CONCLUSIONS
Minor CCT in the CMI patient may cause severe neurological deficit. However, there exists a paucity of reported cases, and the efficacy of suboccipital decompression for these patients remains unclear. Trauma in the cases reviewed, although felt to be minor, often resulted in disproportionate neurologic compromise. We feel that neurologic dysfunction following trauma in those with CMI without syringomyelia or hydrocephalus is multifactorial and determined by the following variables: (1) Age of the patient; (2) Type and severity of trauma, (3) Extent of tonsillar herniation, (4) Dens morphology and angle of retroflexion, and (5) Anatomic variation among individuals in location, proportion, and differential susceptibility to injury of decussating arm and leg corticospinal fibers.
Detailed imaging of the CMJ remains difficult and fiber tract anatomy is not easily obtained. Differentiating edema from contusion in the acute phase post injury is also challenging, but likely to have significant prognostic implications. Advancement of parenchymal MRI techniques about the skull base will allow for better identification and stratification of injury and inform prognosis to correlate neurological deficits with underlying pathoanatomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aboulezz AO, Sartor K, Geyer CA, Gado MH. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: A quantitative approach with MR imaging. J Comput Assist Tomogr. 1985. 9: 1033-6
2. Ajis A, Dharmarajah R. Prolonged recovery of balance after a minor head injury as the only presenting feature of Chiari 1 malformation. Br J Neurosurg. 2004. 73: 398-400
3. Bell HS. Paralysis of both arms from injury of the upper portion of the pyramidal decussation: “Cruciate paralysis”. J Neurosurg. 1970. 33: 376-80
4. Bondurant CP, Oró JJ. Spinal cord injury without radiographic abnormality and Chiari malformation. J Neurosurg. 1993. 79: 833-8
5. Bunc G, Vorsic M. Presentation of a previously asymptomatic Chiari I malformation by a flexion injury to the neck. J Neurotrauma. 2001. 18: 645-8
6. Couldwell WT, Zhang W, Allen R, Acre D, Stillerman CB. Cerebellar contusion associated with type I Chiari malformation following supratentorial head trauma: Case report. Neurol Res. 1998. 20: 93-6
7. Erlich V, Snow R, Heier L. Confirmation by magnetic resonance imaging of Bell's cruciate paralysis in a young child with Chiari type I malformation and minor head trauma. Neurosurgery. 1989. 25: 102-5
8. Fish DR, Howard RS, Wiles CM, Simon L. Respiratory arrest: A complication of cerebellar ectopia in adults. J Neurol Neurosurg Psychiatry. 1988. 51: 714-6
9. Friede RL, Roessmann U. Chronic tonsillar herniation: An attempt at classifying chronic herniations at the foramen magnum. Acta Neuropathol. 1976. 34: 219-35
10. Guo F, Wang M, Long J, Wang H, Sun H, Yang B. Surgical management of Chiari malformation: Analysis of 128 cases. Pediatr Neurosurg. 2007. 43: 375-81
11. Levy WJ, Mason L, Hahn JF. Chiari malformation presenting in adults: A surgical experience in 127 patients. Neurosurgery. 1983. 12: 377-90
12. Mampalam TJ, Andrews BT, Gelb D, Ferriero D, Pitts LH. Presentation of type I Chiari malformation after head trauma. Neurosurgery. 1988. 23: 760-2
13. Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999. 44: 1005-17
14. .editorsNORD Compendium of Rare Diseases and Disorders. New Rochelle, NY: Mary Ann Liebert; 2008. p. 39-
15. Nielson JM.editorsTextbook of Clinical Neurology. New York, NY: Paul B Hoeber; 1947. p. 156-
16. Pappas CE, Gibson AR, Sonntag KH. Decussation of hind-limb and fore-limb fibers in the monkey corticospinal tract: Relevance to cruciate paralysis. J Neurosurg. 1991. 75: 935-40
17. Paul KS, Lye RH, Strang FA, Dutton J. Arnold-Chiari malformation: Review of 71 cases. J Neurosurg. 1983. 58: 183-7
18. Quebada PB, Duhaime AC. Chiari malformation type I and a dolichoodontoid process responsible for sudden cardiorespiration arrest. J Neurosurg. 2005. 103: 567-70
19. Riviello JJ Jr, Marks M, Faerber EN, Steg N. Delayed cervical central cord syndrome after trivial trauma. Pediatr Emerg Care. 1990. 6: 113-7
20. Tomaszek DE, Tyson GW, Bouldin T, Hansen AR. Sudden death in a child with an occult hindbrain malformation. Ann Emerg Med. 1984. 13: 136-8
21. Vleck BW, Ito B. Acute paraparesis secondary to Arnold-Chiari type I malformation and neck hyperflexion. Ann Neurol. 1987. 21: 100-1
22. Wan MJ, Nomura H, Tator CH. Conversion to symptomatic Chiari I malformation after minor head or neck trauma. Neurosurgery. 2008. 63: 748-53
23. Wolf DA, Veasey SP III, Wilson SK, Adame J, Korndorffer WE. Death following minor head trauma in two adult individuals with the Chiari deformity. J Forensic Sci. 1998. 43: 1241-3
24. Zimmerman RA, Wendell GA, Raymond CF.editorsNeuroimaging: Clinical and Physical Principles. New York, NY: Springer-Verlag; 2000. p. 365-411






Julian Carrillo
Posted May 2, 2018, 11:17 am
It would be of interest if you perform a delay MRI to see if the patient develop or not a new cisterna magna