- Department of Neurosurgery and Spine Surgery, Centennial Hospital, Miguel Hidalgo, Aguascalientes, Mexico
- Department of Endovascular Neurosurgery, October 1st Regional Hospital, ISSSTE, Mexico City, Mexico
- Department of Neurosurgery, Juarez Hospital of Mexico, Mexico City, Mexico
- Department of Pediatric Neurosurgery, October 1st Regional Hospital, ISSSTE, Mexico City, Mexico
Correspondence Address:
Aurelio Ponce-Ayala, Department of Neurosurgery and Spine Surgery, Centennial Hospital, Miguel Hidalgo, Aguascalientes, Mexico.
DOI:10.25259/SNI_71_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aurelio Ponce-Ayala1, José de Jesús Gutiérrez-Baños2, Rafael Mendizabal-Guerra3, Mauricio Ivan Rodriguez-Pereira4, Juan Carrizales-Rodriguez3. Chronic subdural hematoma: Clinical experience and recurrence risk factors in a Mexican neurosurgery residency training program. 16-May-2025;16:181
How to cite this URL: Aurelio Ponce-Ayala1, José de Jesús Gutiérrez-Baños2, Rafael Mendizabal-Guerra3, Mauricio Ivan Rodriguez-Pereira4, Juan Carrizales-Rodriguez3. Chronic subdural hematoma: Clinical experience and recurrence risk factors in a Mexican neurosurgery residency training program. 16-May-2025;16:181. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13560
Abstract
Background: Chronic subdural hematoma (CSDH) recurrence remains a challenge, with risk factors still debated.
Methods: A retrospective review of 185 CSDH cases surgically treated at Hospital Juárez de México (2014–2019) was conducted. Recurrence was defined as clinical deterioration with radiological re-expansion. Demographic, clinical, imaging and perioperative variables were analyzed statistically (P
Results: The cohort included 145 males (78.4%) median age of 55 years. Head trauma was present in 75.7%. Hematomas were mostly chronic (69.2%), with 49.18% showing heterogeneous density. The surgical approaches used were single burr-hole (4.3%), double burr-hole (10.8%), and craniotomy (84.9%). Recurrence occurred in 16 cases (8.4%), primarily within a week. The significant risk factors for recurrence included thrombocytopenia (P = 0.001) and prolonged partial thromboplastin time (>38.6 s, P = 0.005). Craniotomy had lower recurrence (7%) than burr-holes (P = 0.053).
Conclusion: Thrombocytopenia and coagulopathy increase recurrence risk in CSDH. Craniotomy may reduce recurrence compared to burr-hole techniques. Further studies are needed to optimize surgical management.
Keywords: Chronic subdural hematoma, Computed tomography, Partial thromboplastin time, Platelet count, Prothrombin time
INTRODUCTION
Chronic subdural hematoma (CSDH) is a collection of blood products in the subdural space (between the dura mater and the arachnoid).[
Figure 1:
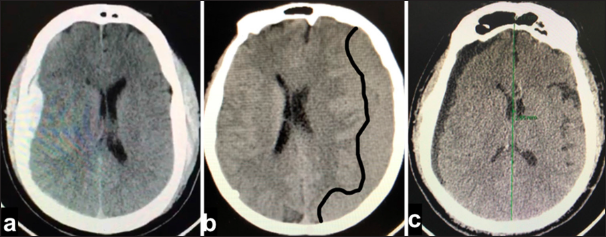
A patient with rapid clinical deterioration in the context of chronic subdural hematoma; (a) edge of skin flap, (b) edge of craniotomy, (c) edge of durotomy, (d) parietal layer of chronic subdural hematoma, (e) visceral layer of chronic subdural hematoma (through this the space can be observed subarachnoid and cerebral cortex), and (f) deteriorating subdural hematoma.
The clinical expression ranges from asymptomatic manifestations such as headache, seizures, visual disturbances, language disorders, incontinence, dysarthria, nausea and vomiting, weakness, ataxia, altered mental status, and coma.[
The diagnostic study of choice is computed tomography (CT) without contrast.[
Figure 3:
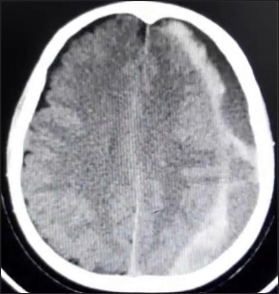
Computed tomography scan of left hemispheric mixed subdural hematoma, the hyperdense image is observed adjacent to the cerebral cortex; lateral to it, we find a hypodense collection; additionally, we observe membranes that go from the hyperdense portion to the dural edge. We did not observe a hematocrit effect [
Figure 4:
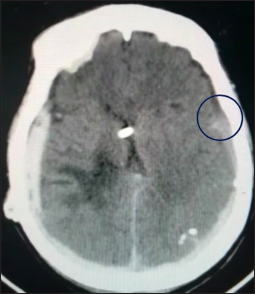
Computed tomography scan showing bilateral subdural hematomas. The blue circle highlights the transition zone between densities within the right-sided hematoma, showing posterior hyperdensity and anterior hypodensity. This pattern is consistent with a chronic-on-acute subdural hematoma, indicating different stages of bleeding and a mixed-age collection.
The therapeutic options are observation,[
Recurrence is a serious complication characterized by a clinical event that reflects deterioration or lack of improvement in neurological function, which is correlated with radiological findings [
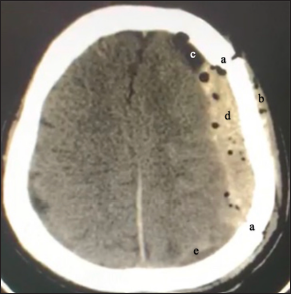
Figure 5:
Computed tomography scan of postoperative acute subdural hematoma in a patient with clinically manifest symptoms (recurrence). Note the suggestive postoperative data; (a) craniotomy site, (b) subcutaneous emphysema, (c) pneumocephalus, (d) associated hyperdense hematoma, and (e) postoperative residual fluid (collection adjacent to the occipital cortex).
This work seeks to illustrate the recurrence in the surgical practice of residents in training at our center and identify potentially modifiable risk factors.
MATERIALS AND METHODS
Of the 288 patients registered in the neurosurgery department of Hospital Juárez de México between January 2014 and January 2019 with a diagnosis of CSDH, 185 files were considered. In this study, we followed the standard classification of subdural hematomas based on their time of evolution and imaging characteristics. Acute subdural hematomas are defined as those <72 h old, presenting as hyperdense collections on CT scans. The subacute phase begins between 3 and 7 days after the initial event, often showing an isodense appearance. CSDHs develop over weeks and are typically hypodense in imaging studies.
We dismissed 103 files because they were either incomplete or absent. Recurrence was defined as clinical deterioration associated with hematoma re-expansion observed on imaging. In cases where no clinical changes were present, radiological criteria alone were sufficient to define recurrence if at least one of the following was met: midline shift ≥5 mm or hematoma volume ≥10 mm.
Descriptive statistical analyses were performed for continuous and categorical variables. Continuous variables are presented as mean ± standard deviation (SD) or median with interquartile range (IQR), depending on their distribution. Specifically, the following values were obtained for the main quantitative variables:
Age (years): mean = 55.0, SD = 0, median = 55.0, IQR = 0.0, blood pressure (BP) at admission (mmHg) ≥140/90: systolic BP (mean = 138.32, SD = 0.47, median = 138.0, IQR = 1.0), postoperative BP (mmHg) ≥140/90: systolic BP (mean = 138.32, SD = 0.47, median = 138.0, IQR = 1.0), platelet count (PLT) (×103/μL): mean = 134.38, SD = 1.65, median = 134.38, IQR = 0.0, prothrombin time (PT, sec): mean = 11.75, SD = 0.43, median = 12.0, IQR = 0.0, partial thromboplastin time (PTT, sec): mean = 35.9, SD = 2.4, and median = 35.5, IQR = 2.0, categorical variables are presented as absolute (n) and relative frequencies (%). The selection of cutoff values for continuous variables (e.g., age ≥60 years, PLT <130,000/μL, PT >11.5 s, and PTT >38.6 s) was based on previously published studies that have used these parameters in CSDH research.[
For the comparison of recurrence rates among different subgroups, we used the Chi-square test or Fisher’s exact test, as appropriate. Continuous variables were analyzed using the Mann–Whitney U-test for non-normally distributed data and the Student’s t-test for normally distributed data. P < 0.05 was considered statistically significant. Statistical analyses were performed using (statistical software, e.g., Statistical Package for the Social Sciences version X.0 [IBM Corp., Armonk, NY, USA]).
Patients who were consumers of oral anticoagulants and antiplatelet drugs underwent a protocol of substituting their drug for enoxaparin 5 days before the intervention. Enoxaparin was suspended 24 h before and restarted 12– 24 h after the procedure. All patients were discharged on the drug that they were taking during admission. In our study, no patients undergoing active antiplatelet or anticoagulation therapy in acute or rapid deterioration scenarios were included for surgical intervention. This exclusion was intentional, as the presence of such pharmacological factors could significantly alter the natural course of the disease and influence the primary outcomes under investigation.
The procedure performed on each patient was selected by the surgeon responsible for the case based on the following characteristics: presence of acute hematoma, presence of hematoma membranes, hematoma segmentation, multiple hematoma densities, midline shift, Glasgow coma scale score at admission, and the neurosurgeon’s experience.
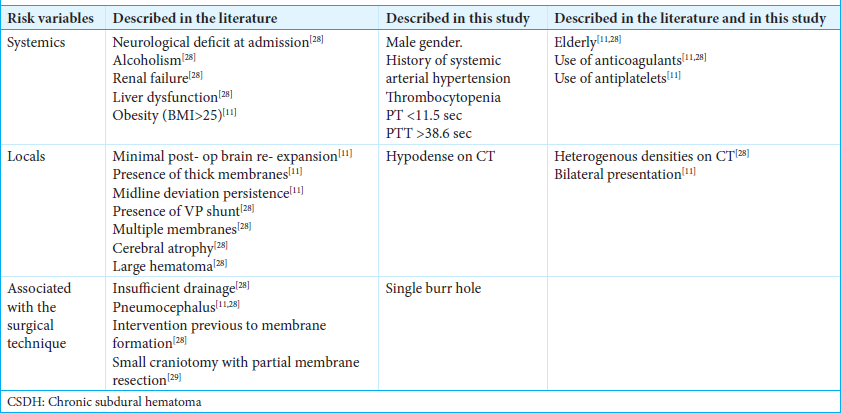
Various systemic, local, and procedure-related factors that increased the recurrence rate in the analyzed studies were considered [
Although we did not systematically classify CSDHs based on their traumatic or non-traumatic origin, most patients (82%) did not report any prior history of head trauma or identifiable impact that could be directly linked to hematoma formation.
Although this study primarily investigates risk factors for CSDH recurrence, we included some acute and subacute cases to evaluate additional variables that may contribute to recurrence. This approach allows for a broader assessment of factors beyond hematoma chronicity, particularly those related to surgical decision-making, the operative technique performed by trainees, and perioperative management.
RESULTS
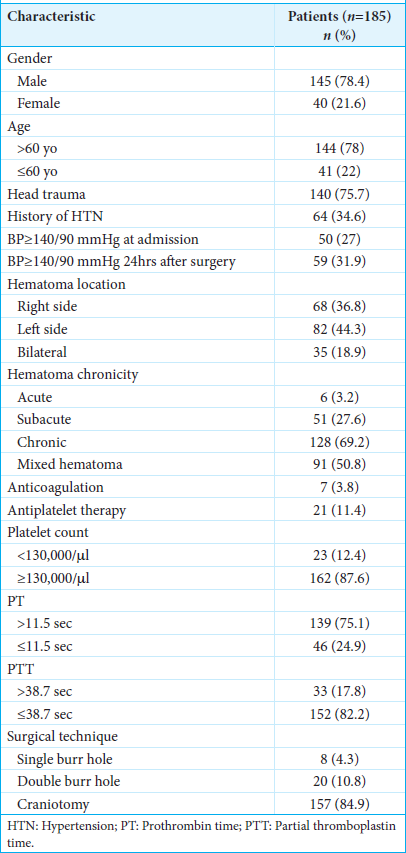
Of the 185 studied patients, 145 (78.4%) were male. Their ages ranged from 19 to 91 years, with 78% of patients above 60 years old. Head trauma was present in 140 (75.7%) patients. We found a history of systemic arterial hypertension in 64 (34.6%) patients, which, in this study, was considered ≥140/90 mmHg. At admission, 50 (27%) patients had BP readings ≥140/90 mmHg, and 59 patients (31.89%) had the same readings within 24 h after surgery. The hematoma appeared on the right side in 68 (36.8%), on the left in 82 (44.3%), and bilaterally in 35 (18.9%) patients. According to the appearance in simple head CT, the hematoma’s chronicity presented the following distribution: acute (hyperdense) in 6 (3.2%), subacute (isodense) in 51 (27.6%), and chronic (hypodense) in 128 (69.2%) patients. Among subacute and chronic hematomas, 91 (49.18%) cases were documented as heterogeneous (mixed). We identified 7 (3.8%) patients under anticoagulation and 21 (11.4%) consuming antiplatelets at admission. During complete blood count and coagulation studies, 23 (12.4%) patients had a PLT <130,000/microliter, 139 (75.1%) showed a preoperative PT >11.5 s, and 33 (17.8%) had a PTT >38.7 s. A single burr-hole was performed in 8 (4.3%) patients and a double burr-hole in 20 (10.8%). The rest of the patients underwent a craniotomy (84.9%).
The characteristics of the patients are shown in
Of the 185 intervened patients, 16 (8.4%) experienced recurrence, with one on the 1st day, six between days 1 and 2, four between days 3 and 4, three between days 5 and 7, one on the 8th day, and one on the 30th day. Among the recurrent patients, 11 (68.8%) were above 60 years old. Subdural drainage was used for over 48 h in all patients except those with acute subdural hematoma diagnoses. No pharmacological treatment was used before, during, or after surgery, aiming for recurrence reduction.
There was no significant difference in recurrence between males (9.7%) and females (5%) with a P = 0.354. Patients with known systemic hypertension had double the recurrence rate compared to those without it, 12.5% and 6.6%, respectively (P = 0.175). There was no significant difference between those who arrived with BP readings >140/90 mmHg and those with <140/90, 8 and 8.8%, respectively (P = 0.849).
Of all the patients studied with CSDH, only 5.17% presented a postoperative hypertensive event (readings above 140/90 mmHg in the postoperative phase) associated with recurrence, proving not to be a determinant risk factor (P = 0.238). Regarding the location of the hematoma, those with a bilateral component recurred 17.14% of the time, as opposed to 7.35% (right) and 6.09% (left) when they were unilateral (P = 0.205).
According to the CT densities, we observed that hypodense (chronic) hematomas had a greater recurrence rate (9.37%) than isodense (subacute) and hyperdense (acute) hematomas, which had rates of 7.84% and 0%, respectively (P = 0.552).
When dealing with heterogeneous hematomas, we observed a recurrence rate of 10.98% compared to 6.38% for homogeneous hematomas (P = 0.498).
The group that consumed oral anticoagulants had a higher recurrence rate (14.28%) than the group that did not (8.42%) (P = 0.589). Patients on antiplatelet therapy had a lower recurrence rate than those who did not, with rates of 4.76% and 9.14%, respectively (P = 0.501).
The group of patients considered to have thrombocytopenia (according to local laboratory parameters: <130,000 × µL) had a recurrence rate of 26.08%. In contrast, the group of patients with higher readings showed a recurrence rate of 6.17% (P = 0.001). No patient entered the operating room with values lower than 80,000 × μL (five had lower values and received transfusions until their values reached >80,000 × μL).
The group of patients with PT >11.6 s had a recurrence rate of 9.35% compared to those with lower readings (P = 0.554). The group of patients with PTT >38.6 s showed a recurrence rate of 21.21%, which was significantly different than that of patients with shorter time (5.92%) (P = 0.005).
Regarding the variety of surgical techniques, the single burr-hole technique had a recurrence rate of 25%, the double burr-hole technique had a recurrence rate of 15%, and craniotomy had a recurrence rate of 7%, almost reaching statistical significance (P = 0.053). In this study, we rarely used the single burr-hole technique (4.3%).
DISCUSSION
CSDH is a common type of intracranial hemorrhage that primarily affects elderly individuals. Several risk factors have been associated with the development and recurrence of CSDH.
Concerning the risk factors associated with CSDH recurrence, we observed some demographic similarities with those described in the literature, such as advanced age and gender.[
Nevertheless, it remains a point of interest whether residual estrogenic activity, prior lifetime exposure, or hormone replacement therapy could play a role in modifying recurrence risk even in postmenopausal women. Further studies focusing on the hormonal milieu in elderly female patients with CSDH would be required to elucidate the precise impact of estrogen depletion on recurrence rates.[
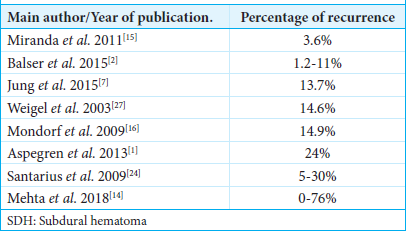
Our service’s overall recurrence rate was 8.64%, which falls within the range presented in the world literature.[
In our study, we found that hypodense hematomas in CT showed a higher recurrence rate (9.37%), which differs from other cohorts found in the literature that demonstrated a higher incidence in isodense and single-layer hematomas.[
Although our series demonstrated a higher recurrence rate with the double burr-hole technique (15%) compared to craniotomy (7%), multiple international studies have identified the double burr-hole procedure as the most effective technique for CSDH drainage.[
Based on the data obtained through inferential statistics, we can identify some risk factors that, although they did not show statistical significance if modified, could hypothetically reduce the risk of recurrences, such as the surgical technique (the limited sample size could represent a significant bias when interpreting the results) and the use of anticoagulants. Moreover, our cohort found that a PLT <130,000 and PTT >38.6 showed statistical significance as risk factors for recurrence. Hence, we strongly recommend that more careful measures be taken with this group of patients. Specifically, patients on antiplatelet therapy (e.g., aspirin and clopidogrel) or anticoagulation therapy (e.g., warfarin and direct oral anticoagulants) require distinct preoperative management strategies, such as platelet transfusion or administration of procoagulant agents (e.g., prothrombin complex concentrates or Vitamin K antagonists). These interventions, aimed at reversing the anticoagulant effects, could confound our assessment of hematoma recurrence and the impact of surgical technique. Given our study’s focus on evaluating recurrence rates under standard perioperative conditions, we opted to exclude this subgroup to maintain homogeneity in our patient population and avoid potential confounding variables.
Other readily available measures that could impact the reduction of recurrence, morbidity, and mortality include the use of drainage and preoperative administration of drugs such as steroids.[
In addition, acute alcohol intoxication has been reported in the literature as a potential risk factor for CSDH recurrence due to its transient effects on platelet function and coagulation pathways. However, in our current study, we did not systematically evaluate acute alcohol intoxication as a distinct risk factor. We acknowledge that this variable may have influenced our findings and recognize the importance of addressing it in future research to further clarify its role in the risk of recurrence.
At present, there are studies focused on assessing the scope of endovascular therapy to facilitate the reabsorption of the collection through the embolization of the middle meningeal artery.[
CONCLUSION
This study shows a statistically significant association between recurrence and the patient’s and the hematoma’s characteristics. For this reason, this paper will support the conduction of prospective studies that solidly support any recommendation to reduce the recurrence rate.
As far as we are concerned, our patients’ prognoses can improve if we modify some of the risks identified here, especially the PLT and PTT. Although no statistical significance was reached, we recommend the double burr-hole technique for the surgical management of this neurological entity.
Ethical approval:
The Institutional Review Board of Hospital Juárez de México approved this study (Approval Number: HJM 0667/19-R; Date: July 28, 2020) in compliance with all ethical guidelines for human research.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment:
We thank everyone involved in the process of this research project.
References
1. Aspegren OP, Åstrand R, Lundgren MI, Romner B. Anticoagulation therapy a risk factor for the development of chronic subdural hematoma. Clin Neurol Neurosurg. 2013. 115: 981-4
2. Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans administration and civilian populations. J Neurosurg. 2015. 123: 1209-15
3. Delgado-López PD, Martín-Velasco V, Castilla-Díez JM, Rodríguez-Salazar A, Galacho-Harriero AM, FernándezArconada O. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia (Astur). 2009. 20: 346-59
4. Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: Epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. 2020. 141: 339-45
5. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural hematoma: Surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005. 107: 223-9
6. Haines DE, Harkey HL, Al-Mefty O The. “subdural” space: A new look at an outdated concept. Neurosurgery. 1993. 32: 111-20
7. Jung YG, Jung NY, Kim E. Independent predictors for recurrence of chronic subdural hematoma. J Korean Neurosurg Soc. 2015. 57: 266-70
8. Kageyama H, Toyooka T, Tsuzuki N, Oka K. Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg. 2013. 119: 332-7
9. Kim J, Moon J, Kim T, Ahn S, Hwang G, Bang J. Risk factor analysis for the recurrence of chronic subdural hematoma: A review of 368 consecutive surgical cases. Korean J Neurotrauma. 2015. 11: 63-9
10. Kim JH, Kang DS, Kim JH, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 2011. 50: 103-8
11. Lee KS. History of chronic subdural hematoma. Korean J Neurotrauma. 2015. 11: 27-34
12. Lee KS. Natural history of chronic subdural hematoma. Brain Inj. 2004. 18: 351-8
13. Markwalder TM, Steinsiepe KF, Rohner M, Reichenbach W, Markwalder H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981. 55: 390-6
14. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: A review of the literature. J Clin Neurosci. 2018. 50: 7-15
15. Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: Not a benign disease. J Neurosurg. 2011. 114: 72-6
16. Mondorf Y, Abu-Owaimer M, Gaab MR, Oertel JM. Chronic subdural hematoma--craniotomy versus burr hole trepanation. Br J Neurosurg. 2009. 23: 612-6
17. Naganuma H, Fukamachi A, Kawakami M, Misumi S, Nakajima H, Wakao T. Spontaneous resolution of chronic subdural hematomas. Neurosurgery. 1986. 19: 794-8
18. Parizel PM, Makkat S, Van Miert E, Van Goethem JW, Van Den Hauwe L, De Schepper AM. Intracranial hemorrhage: Principles of CT and MRI interpretation. Eur Radiol. 2001. 11: 1770-83
19. Parlato C, Guarracino A, Moraci A. Spontaneous resolution of chronic subdural hematoma. Surg Neurol. 2000. 53: 312-5 discussion 315-7
20. Quiñones-Hinojosa A, editors. Schmidek and Sweet: Operative Neurosurgical Techniques. Netherlands: Elsevier; 2012. p.
21. Rust T, Kiemer N, Erasmus A. Chronic subdural hematomas and anticoagulation or anti-thrombotic therapy. J Clin Neurosci. 2006. 13: 823-7
22. Sambasivan M. An overview of chronic subdural hematoma: Experience with 2300 cases. Surg Neurol. 1997. 47: 418-22
23. Santarius T, Hutchinson PJ. Chronic subdural hematoma: Time to rationalize treatment?. Br J Neurosurg. 2004. 18: 328-32
24. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P. Use of drains versus no drains after burr-hole evacuation of chronic subdural hematoma: A randomised controlled trial. Lancet. 2009. 374: 1067-73
25. Suzuki J, Takaku A. Nonsurgical treatment of chronic subdural hematoma. J Neurosurg. 1970. 33: 548-53
26. Thotakura AK, Marabathina NR. The role of medical treatment in chronic subdural hematoma. Asian J Neurosurg. 2018. 13: 976-83
27. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural hematoma: Evidence based review. J Neurol Neurosurg Psychiatry. 2003. 74: 937-43
28. Winn HR, editors. Youmans and Winn neurological surgery. Netherlands: Elsevier; 2017. p.