- Department of Neurosurgery, Izumi Regional Hospital, Akune, Japan
- Department of Neurosurgery, Kagoshima University, Kagoshima, Japan
- Sakuradori Clinic, Izumi, Japan
- Department of Neurology, Imamura General Hospital, Kagoshima, Japan
Correspondence Address:
Kazunori Arita, Department of Neurosurgery, Izumi Regional Hospital, Akune, Japan.
DOI:10.25259/SNI_153_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kazunori Arita1, Koshi Yokota2, Yushi Nagano2, Hitoshi Yamahata2, Nayuta Higa2, Masaaki Yamamoto3, Junpei Kubo4, Ryosuke Hanaya2. Classical type of superficial hemosiderosis presenting with temporal lobe epilepsy. 06-Jun-2025;16:225
How to cite this URL: Kazunori Arita1, Koshi Yokota2, Yushi Nagano2, Hitoshi Yamahata2, Nayuta Higa2, Masaaki Yamamoto3, Junpei Kubo4, Ryosuke Hanaya2. Classical type of superficial hemosiderosis presenting with temporal lobe epilepsy. 06-Jun-2025;16:225. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13614
Abstract
Background: Classical type of superficial hemosiderosis (SH) is subpial hemosiderin deposition mainly affecting the cerebellum, brainstem, and spinal cord, which generally presents with cerebellar ataxia and sensorineural hearing disturbance. We here report a rare case of the classical type of SH presenting with temporal lobe epilepsy and perform a literature review on similar cases.
Case Description: A 63-year-old man with four episodes of impaired awareness and confusion lasting for around 5 minutes after feeling vague uneasiness, suggesting focal impaired awareness seizure, visited a neurosurgical clinic. T2*-weighted magnetic resonance imaging (MRI) showed hemosiderin deposition on the surface of the cerebellum, brainstem, upper spinal cord, and bases of bilateral frontal and temporal lobes. Neurological examination found mild gait ataxia and anosmia. Audiogram showed sensorineural high-frequency hearing loss. Electroencephalogram showed rhythmic theta activities accompanied by intermittent sharp waves over the right fronto-temporal region during a subclinical seizure episode, which led to the diagnosis of temporal lobe epilepsy. Up-dosing of levetiracetam to 1,500 mg/day brought about a seizure-free status. Gait disturbance, however, gradually deteriorated over the following 6 months. Spinal MRI and myelogram found a dural defect at the T3 level. The 4 mm long defect was surgically closed, which led to the gradual improvement of the gait ataxia.
Conclusion: In this case of the classical type of SH due to a dural defect, temporal lobe epilepsy is presumably caused by the neurotoxicity of decomposed products of hemoglobin impregnated in the temporal lobes.
Keywords: Anosmia, Ataxia, Hearing disturbance, Superficial hemosiderosis, Temporal lobe epilepsy
INTRODUCTION
Superficial hemosiderosis (SH) is a central nervous system (CNS) disease caused by subpial hemosiderin deposition secondary to chronic hemorrhage in the subarachnoid space.[
CASE DESCRIPTION
An otherwise healthy 63-year-old man, a farmer, visited a neurosurgical clinic with four episodes of impaired awareness and confusion lasting for around 5 minutes after feeling vague uneasiness. He looked around during these episodes. He also had some 10-minute-long memory disturbances after the episodes. No apparent automatic movements during the episodes were reported. The episodes suggested that he had focal impaired awareness seizures (FIAS) attacks. Abnormalities found on magnetic resonance imaging (MRI) at the clinic urged further examination at our neurosurgical center. Neurological examination showed mild cerebellar ataxia, hearing disturbance, anosmia, and taste impairment. Audiogram showed bilateral sensorineural high-frequency hearing loss. The Mini-Mental State Examination score was 28/30. T2*-weighted MRI found thick deposition of hemosiderin on the surface of the cerebellum, brainstem, upper spinal cord, and bases of the bilateral frontal and temporal lobes [
Figure 1:
T2*-weighted magnetic resonance imaging of the brain and the upper cervical cord at the presentation. (a-e) It showed low-intensity rims, hemosiderin deposition, on the infratentorial structures including cerebellum and the base of supratentorial structures including the temporal lobes. (f) It also showed hemosiderin deposition on the surface of the upper cervical cord.
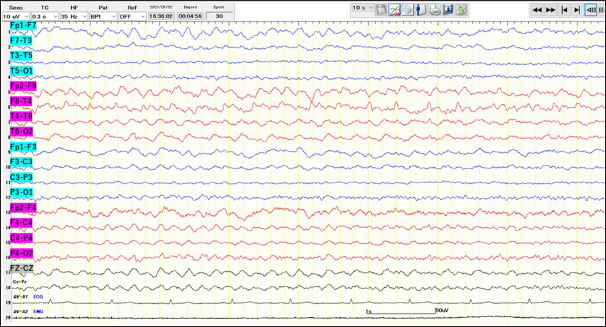
Figure 2:
Longitudinal bipolar electroencephalography during light sleep showed high-amplitude rhythmic theta activity in the right fronto-temporal region, accompanied by intermittent sharp waves maximal at F8, suggesting a subclinical seizure. Blue lines are from the left-side electrodes. Red lines are from the right-side electrodes.
He was given an increasing dose of levetiracetam up to 1,500 mg/day, which prevented further epilepsy attacks. However, gait disturbance slowly progressed, eventually disturbing even daily activities. Sagittal T2*-weighted spinal MRI showed the deposition of hemosiderin over the upper thoracic spinal cord [
Figure 3:
Neuroimaging of the thoracic spine. (a) T2*-weighted midline sagittal magnetic resonance imaging (MRI) of the thoracic spine showed hemosiderin deposition on the upper thoracic spinal cord and osteophytes at the T3-T4 and T4-T5 disc levels. (b, c) T2*-weighted off-midline sagittal MRI showed fluid collection outside the dura mater at the T1-T6 level (arrows). (d, e) Axial constructive interference in steady state-weighted MRI showed a left antero-lateral extradural fluid collection at the T3 and T4 levels (arrow). It also showed a dural defect at the T3 level (arrowhead in d). (f) A computer tomographic myelogram showed that the contrast material in the cerebrospinal fluid leaked into the extradural space (arrow).
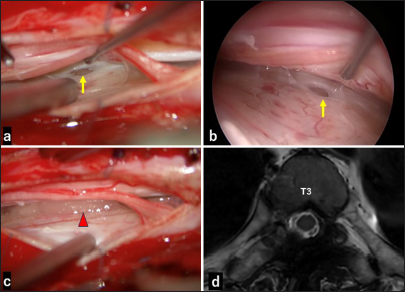
Figure 4:
Intraoperative exploration and postoperative magnetic resonance imaging (MRI). (a) Microscopic exposure at the lower T3 level showed the dural defect (arrow). (b) Endoscopic observation confirmed the defect (arrow). (c)The defect was sealed using collagen matrix grafts and a stitch (arrowhead). (d) Postoperative axial constructive interference in steady state-weighted MRI revealed the disappearance of the extradural cerebrospinal fluid collection.
The unsteadiness of the gait gradually improved since the surgery. He returned to rice farming 4 months after the surgery. FIAS attacks have not recurred for the past 19 months.
Literature review
In almost all large case series and past literature reviews, seizures were not mentioned as a symptom of SH.[
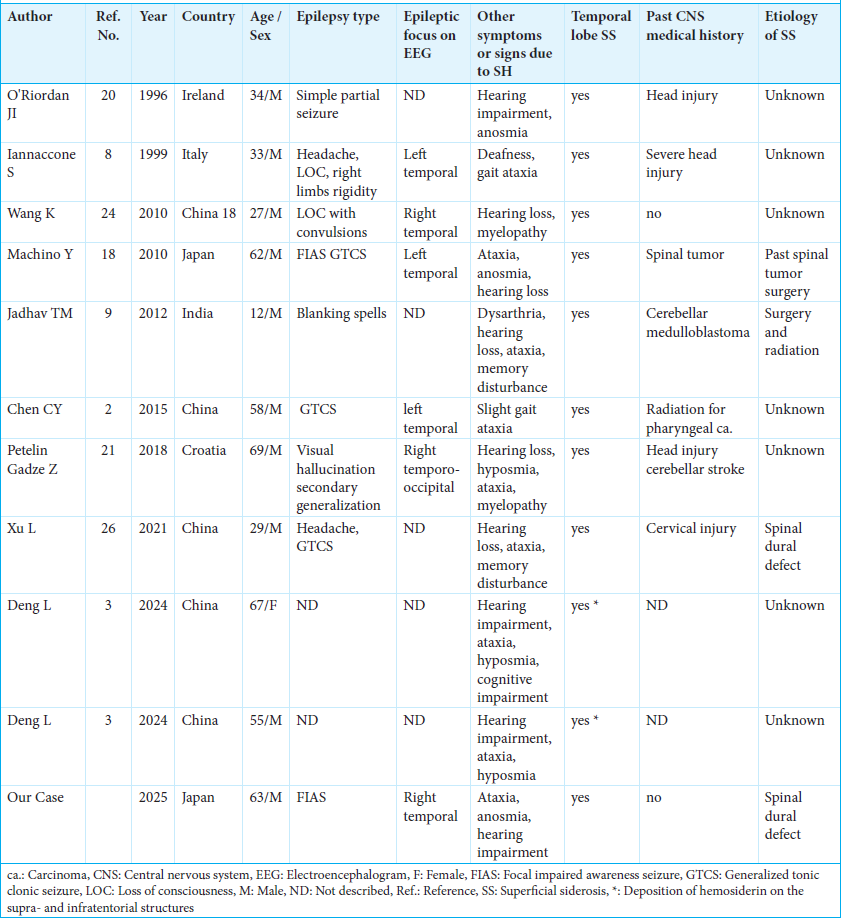
Our literature retrieval, however, found 11 reported cases (ten males and one female), including ours, in which seizures are considered to be due to SH [
The median age of these 11 patients was 55 years old (range: 12–69). Partial seizures, described as simple partial seizures, FIAS, headache, visual hallucination, and blanking spells, were seen in six. Convulsive seizures, described as generalized tonic-clonic seizures, loss of consciousness with convulsion, limb rigidity, and secondary generalization, were seen in six. The epileptic focus on EEG was in the temporal leads in six patients, and not described in five. SH on the temporal lobes on MRI was observed in all patients. At least one classical symptom of SH, such as hearing impairment and gait ataxia, was recorded in all patients. Past medical history regarding CNS disease was recorded in six patients, including surgeries on CNS tumors, head injuries, cervical injury, cerebellar stroke, and radiation. The cause of SH was reported to be a spinal dural defect in two patients, past spinal tumor surgery in one, past treatment for cerebellar medulloblastoma in one, and unknown in the other seven.
DISCUSSION
This is a case of SH who initially presented with FIAS or complex partial seizure. EEG showed a right temporal focus. Spinal MRI found a T3 level dural defect, the surgical closure of which led to a gradual improvement of ataxia.
SH is a rare disorder characterized by subpial diffuse hemosiderin deposition caused by prolonged, slow subarachnoid hemorrhage on the surface of the brain and spinal cord.[
However, our literature survey found 11 reported cases, including ours, of SH presenting with seizures.[
A recent report showed that post-stroke cerebral SH is far more common in patients with post-stroke epilepsy (PSE) than in patients without PSE.[
Although secondary or type 2 SH shows local hemosiderin deposition around the bleeding site due to various pathologies,[
CONCLUSION
We reported a rare case of SH presenting with a simple partial seizure with a right temporal focus. An anti-epileptic drug has well-controlled the seizures. He also showed common symptoms of SH, such as hearing disturbance and gait ataxia. The progression of gait ataxia ceased after the repair of a dural tear at the T3 level. This report stresses the importance of meticulous history taking about symptoms suggesting partial seizures in SH patients and searching for the dural tear in the spinal canal for the quick and proper treatment of SH. In addition, this report suggests the routine check-up of brain hemosiderosis, including SH, using T2*-weighted MRI to find the etiology in patients with simple partial seizures.
Ethical approval:
The Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment:
The authors would like to thank Professor Koji Iida, Department of Epileptology, Hiroshima University Hospital, for the EEG reading and Haibunsha Kagoshima for performing a critical review of this manuscript and English language editing.
References
1. Arishima H, Higashino Y, Yamada S, Akazawa A, Arai H, Tsunetoshi K. Spinal endoscopy combined with selective CT myelography for Dural closure of the spinal Dural defect with superficial siderosis: Technical note. J Neurosurg Spine. 2018. 28: 96-102
2. Chen CY, Xiao F, Liu JL. Superficial siderosis of the central nervous system with seizures onset. Singapore Med J. 2015. 56: 590-1
3. Deng L, Lin Y, Lin Y, Huang W. Infratentorial superficial siderosis: Report of six cases and review of the literature. Front Neurosci. 2024. 18: 1373358
4. Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain. 1995. 118: 1051-66
5. Grover N, Whiteside OJ, Ramsden JD. Cochlear implantation in superficial siderosis: A viable option?. Cochlear Implants Int. 2011. 12: 241-3
6. Hashimoto M, Egawa S, Hirai T, Hashimoto J, Morishita S, Yamada K. Detection of Dural defect localization using 4-dimensional dynamic computed tomography myelography for patients with superficial siderosis. World Neurosurg. 2024. 187: e798-806
7. Hirano T, Enatsu R, Iihoshi S, Mikami T, Honma T, Ohnishi H. Effects of hemosiderosis on epilepsy following subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2019. 59: 27-32
8. Iannaccone S, Golzi V, Sferrazza B, De Rino F, Smirne S, Ferini-Strambi L. Central nervous system superficial siderosis, headache, and epilepsy. Headache. 1999. 39: 666-9
9. Jadhav TM, Hegde AU. Superficial siderosis: A rare occurrence in children. J Pediatr Neurosci. 2012. 7: 215-7
10. Koeppen AH, Michael SC, Li D, Chen Z, Cusack MJ, Gibson WM. The pathology of superficial siderosis of the central nervous system. Acta Neuropathol. 2008. 116: 371-82
11. Kumar N, Cohen-Gadol AA, Wright RA, Miller GM, Piepgras DG, Ahlskog JE. Superficial siderosis. Neurology. 2006. 66: 1144-52
12. Kumar N. Neuroimaging in superficial siderosis: An in-depth look. AJNR Am J Neuroradiol. 2010. 31: 5-14
13. Kumar N. Beyond superficial siderosis: Introducing “Duropathies”. Neurology. 2012. 8: 1992-9
14. Kumar N. Superficial Siderosis: A clinical review. Ann Neurol. 2021. 89: 1068-79
15. Leussink VI, Flachenecker P, Brechtelsbauer D, Bendszus M, Sliwka U, Gold R. Superficial siderosis of the central nervous system: Pathogenetic heterogeneity and therapeutic approaches. Acta Neurol Scand. 2003. 107: 54-61
16. Levy M, Turtzo C, Llinas RH. Superficial siderosis: A case report and review of the literature. Nat Clin Pract Neurol. 2007. 3: 54-8 quiz 59
17. Lummel N, Wollenweber FA, Demaerel P, Bochmann K, Malik R, Opherk C. Clinical spectrum, underlying etiologies and radiological characteristics of cortical superficial siderosis. J Neurol. 2015. 262: 1455-62
18. Machino Y, Nakayama S, Takashima S, Tomimoto H. A case of superficial siderosis with repeated episodes of epilepsy. Rinsho Shinkeigaku. 2010. 50: 108-10
19. Ohira M, Takao M. Nationwide epidemiological survey of superficial hemosiderosis in Japan. J Neurol Sci. 2019. 404: 106-11
20. O’Riordan JI, Javed M, McShane D, Hutchinson M, Murphy R. Superficial siderosis of the central nervous system. Ir J Med Sci. 1996. 165: 182-4
21. Petelin Gadze Z, Milat D, Derke F, Bosnjak Pasic M, Bilic E. Epilepsy caused by superficial hemosiderosis of the central nervous system. Neurol Sci. 2018. 39: 781-3
22. Takai K, Taniguchi M. Superficial siderosis of the central nervous system associated with ventral Dural defects: Bleeding from the epidural venous plexus. J Neurol. 2021. 268: 1491-4
23. Tanaka T, Fukuma K, Abe S, Matsubara S, Ikeda S, Kamogawa N. Association of cortical superficial siderosis with post-stroke epilepsy. Ann Neurol. 2023. 93: 357-70
24. Wang K, Xu Z, Xiong G, Benyan L. Superficial siderosis of the central nervous system manifested with seizures. J Clin Neurosci. 2010. 17: 277-8
25. Wilson D, Chatterjee F, Farmer SF, Rudge P, McCarron MO, Cowley P. Infratentorial superficial siderosis: Classification, diagnostic criteria, and rational investigation pathway. Ann Neurol. 2017. 81: 333-43
26. Xu L, Yuan C, Wang Y, Shen S, Duan H. Superficial siderosis of the central nervous system with epilepsy originating from traumatic cervical injury: Illustrative case. J Neurosurg Case Lessons. 2021. 1: CASE2114