- Department of Neurosurgery, Desert Regional Medical Center, Palm Springs, California,
- School of Medicine, University of New Mexico, Albuquerque, New Mexico,
- College of Osteopathic Medicine, Michigan State University, East Lansing, Michigan,
- College of Osteopathic Medicine, Western University of Health Sciences, Pomona, California,
- College of Osteopathic Medicine, William Carey University, Hattiesburg, Mississippi, United States.
Correspondence Address:
Brian Fiani
College of Osteopathic Medicine, William Carey University, Hattiesburg, Mississippi, United States.
DOI:10.25259/SNI_133_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brian Fiani1, Juliana Runnels2, Alexander Rose2, Athanasios Kondilis3, Amelia Wong4, Brian L. Musch5. Clinical manifestations, classification, and surgical management of sacral tumors and the need for personalized approach to sacrectomy. 03-May-2021;12:209
How to cite this URL: Brian Fiani1, Juliana Runnels2, Alexander Rose2, Athanasios Kondilis3, Amelia Wong4, Brian L. Musch5. Clinical manifestations, classification, and surgical management of sacral tumors and the need for personalized approach to sacrectomy. 03-May-2021;12:209. Available from: https://surgicalneurologyint.com/surgicalint-articles/10775/
Abstract
Background: Although comprising 7% of all spinal tumors, sacral tumors present with a litany of issues due to their slow growth and difficulty in detection. As a result, sacral tumors can grow unperturbed for years until a patient presents for an incidental workup of an unassociated minor trauma or an offending primary tumor source that has metastasized to the sacrum; in most cases, this includes primary tumors of the breast, prostate, and lung. The goal of this review is to outline the pathophysiology underlying sacral tumors including the various tissues and structures that can be targeted for treatment, along with a discussion of the surgical approach to sacrectomy.
Methods: An extensive review of the published literature was conducted through PubMed database with articles simultaneously containing both search terms “sacral tumors” and “sacrectomy.” No date restrictions were used.
Results: The search yielded 245 related articles. Cross-checking of articles was conducted to exclude of duplicate articles. The articles were screened for their full text and English language availability. We finalized those articles pertaining to the topic.
Conclusion: Once a sacral tumor has reached the point of diagnostic detection, invasive sacrectomy is typically utilized (through an anterior, posterior, or combination approach) to locally isolate and resect the tumor and minimize risk of future tumor growth and additional bone loss. While institutions have varying criteria for surgical approaches, a combination of anterior and posterior approach has traditionally been used in total and high sacrectomies due to the control it provides surgeons toward the rectum and vasculature anterior to the sacrum. A posterior-only approach can be performed for tumors that failed to invade pelvic organs or extend past the lumbosacral junction. Early detection with screenings can help avoid invasive sacrectomy by identifying the onset of tumor formation in the sacrum, particularly for highly metastatic cancers.
Keywords: Bone metastasis, Sacral tumor, Sacrectomy, Sacrum, Spinal tumor
INTRODUCTION
Sacral tumors are rare slow-growing lesions, accounting for less than 7% of all spinal tumors. Many cases remain clinically silent and are incidentally discovered during workup of minor trauma.[
The purpose of this review is to examine the pathophysiology of sacral tumors; particularly as a result of clinical manifestations including patient presenting signs and symptoms. The difficulty posed by sacral tumors (and what this review aims to accomplish) from a clinical perspective is to identify unique characteristics of the various sacral tumors instead of the nonspecific and commonly overlapping symptoms presented clinically. This review will focus on the tumors that are primarily localized to the sacrum, however, metastatic lesions to the sacrum will also be described in detail. Further, surgical interventions for the treatment of various sacral tumors will be described (including anterior and posterior approaches) as well as recommended postoperative management to minimize risk of recurrence and other adverse events. The objective is to identify genetic markers, patient presentations, radiographic imaging, histological features, and other highly sensitive and specific tests that may guide the diagnosis, staging, and treatment of sacral tumors regardless of tissue origin or severity.
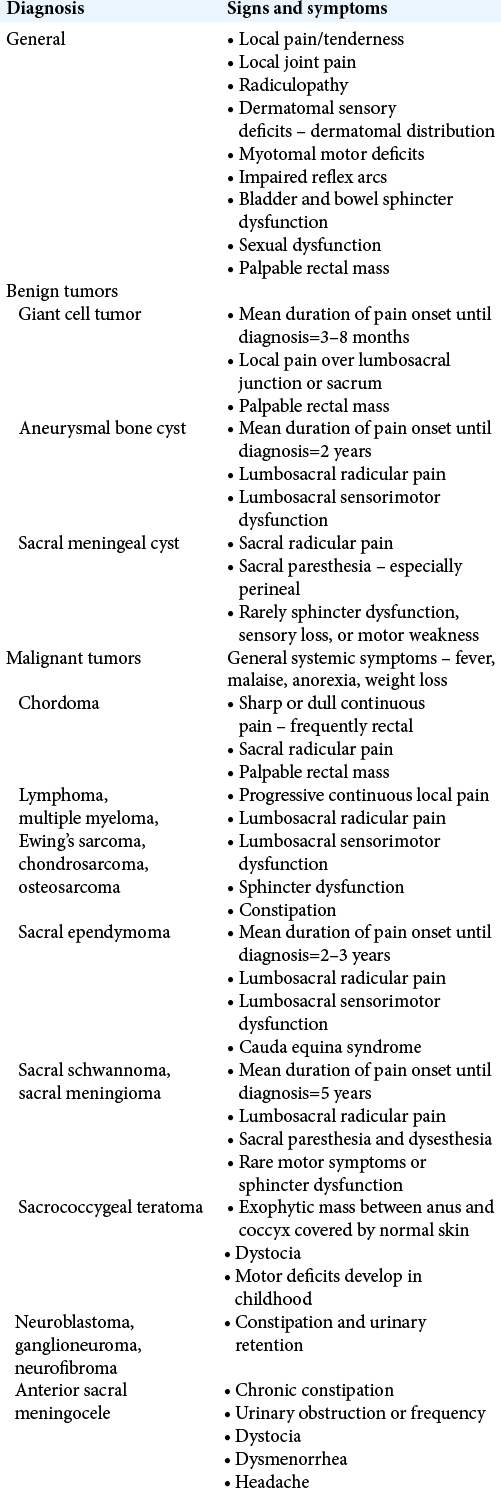
SIGNS AND SYMPTOMS
While some sacral tumors are associated with specific demographics or manifestations, they largely exhibit similar signs and symptoms, thus making it difficult or impossible to diagnose the tumor type based on clinical presentation alone [
TYPES OF SACRAL TUMORS
Primary bone tumors can be differentiated by histologic origin. The most widely adopted pathologic classification system for bone tumors is the World Health Organization classification system [
The benign and malignant pathologies that can present at in the sacral level are further classified as metastatic disease, congenital tumors, primary bone tumors, or primary neurogenic tumors, as outlined in [
Metastasis
Osseous metastasis is one of the most frequent and debilitating manifestations of advanced cancer. Metastatic bone tumors are more common than primary bone tumors.[
Congenital tumors
Teratoma
The most common sacral tumors in the pediatric population are sacrococcygeal teratoma.[
Hamartoma
Congenital spinal hamartomas are comprised of well-differentiated mesodermal and ectodermal tissue.[
Dermoid cyst
As the neural groove begins to seal between the 3rd and 5th weeks of embryonic life, inclusion of ectodermal elements can form a dermoid cyst.[
Perineural cyst
Also referred to as Tarlov cysts, perineural cysts are caused by meningeal dilations of the spinal nerve root sheath. Perineural cysts involving the sacrum can lead to profound bony erosion, and cases of resultant compression fracture have been reported.[
Meningocele
Anterior meningocele is caused by herniation of the dural sac through a sacral defect. The pathognomonic radiographic finding is the “scimitar sign,” which describes a sacrum with a round, concave border devoid of any destruction.[
Primary bone tumors
Chordoma
Chordomas are the most common primary tumor of the sacrum and arise from notochordal tissue. Chordomas, which arise from notochordal remnants in the sacrum, are the most common malignant primary sacral tumor.[
Aneurysmal bone cyst (ABC)
ABCs are benign tumors that can be locally aggressive. Large lesions can cause mass effect or pathologic fracture. Imaging often reveals an expansile lytic lesion with a thin calcific rim and characteristic multiloculated spaces with fluid levels.[
Giant cell tumor
Giant cell tumors predominantly manifest in the extremities, but most often occur at the sacrum when involving the axial skeleton. Although primary giant cell tumors are histologically benign, metastases to the lung have occasionally been reported.[
Lymphoma
Primary lymphoma of the bone is a rare round cell malignancy that can be locally destructive. T1-weighted images show an ill-defined soft-tissue mass.[
Multiple myeloma
Unifocal multiple myeloma manifests as a solitary osseous plasmacytoma. On radiograph or CT, solitary plasmacytoma appears as an expansive lytic mass with peripheral sclerosis. T1-weighted MRI demonstrates low signal intensity, and T2-weighted images will display postcontrast enhancement.[
Ewing’s sarcoma
Ewing’s sarcoma occurs most frequently in young males. On imaging, Ewing’s sarcoma appears as an osteolytic lesion with soft-tissue component. The lesion commonly appears as a homogenous hypointense signal on T1-weighted images and an isointense signal on T2-weighted MR.
Chondrosarcoma
Chondrosarcomas are associated with a lobular appearance on imaging and pathology commonly demonstrates lobules and chondroid matrix. Characteristic radiographic finding is an osteolytic right sacral mass with a soft-tissue component and intratumoral chondroid-type calcifications.[
Osteosarcoma
Osteosarcomas account for approximately 4% of all sacral primary bone tumors. Sunburst calcifications are characteristic on imaging and spindle cells with osteoid matrix characteristic on biopsy.[
Chondromyxoid fibroma (CMF) of the sacrum
CMFs of the sacrum are a rare benign cartilaginous tumor that histologically is characterized by hypochromic lobules of stellate or spindle-shaped cells. The tumor stains positive for S-100, Sox 9, and Type II collagen.[
Primary neurogenic tumors
Schwannoma
Schwannomas are associated with a characteristic appearance on imaging. MR reveals a large, well-defined heterogeneous mass that may be associated with minor underlying erosion. Cystic formation, hemorrhage, and necrosis may also be apparent. In contrast to neurofibromas, schwannomas are encapsulated.[
Neurofibroma
Neurofibroma originates in nerve fascicles comprised of Schwann cells, fibroblasts, mast cells, and axons. Neurofibromas appear radiolucent and well circumscribed on imaging. Biopsy is characterized by short spindle cells with long, wavy nuclei that stain positive for S100.[
Staging
At present, two systems are available for staging primary malignant bone tumors – the Musculoskeletal Tumor Society System (MSTS) and the American Joint Committee on Cancer (AJCC) system. The staging system adopted by the MSTS was first described by Enneking et al. in 1980 and was based on three criteria: extent of tumor, metastasis, and grade.[
Table 4:
Enneking staging system for primary malignant tumors of the bone.[
Table 5:
The 8th Edition AJCC staging system for primary malignant tumors of bone.[
SURGICAL APPROACHES
Optimal surgical technique typically prefers wide surgical margins because it prevents incomplete resection that can lead to local regrowth. A wide tumor resection includes a continuous encasement of healthy tissue around the tumor.[
While institutions have varying criteria for surgical approaches, a combination of anterior and posterior approach has traditionally been used in total and high sacrectomies due to the control it provides surgeons toward the rectum and vasculature anterior to the sacrum [
Institutions have used a posterior-only approach in middle, low, and distal sacrectomies, as an anterior-posterior approach shows itself to be implausible.[
A sacrifice of nerve roots with functional impairment presents itself in both partial and complete sacrectomies, but this sacrifice is necessary to achieve proper local control.[
POSTOPERATIVE MANAGEMENT
The most common complaint after sacrectomy is sacral pain. Average duration of pain is 8 months with 15% reported risk of neuropathic pain and complex regional pain syndrome.[
Surgical site infection and wound dehiscence are also common complications after sacrectomy. Enteral feeding is conventionally associated with improved postoperative nutritional and immunologic status. However, operative disruption sacral nerve roots leading to bladder and bowel dysfunction increases the risk of infection as fecal leak can contaminate surgical wounds and provide a nidus for infection. The most common bacterial pathogens implicated are Enterococcus (23%) and Escherichia coli (20%). To abate this risk, Gao et al. endorsed early postoperative fasting and total parenteral nutrition while others may elect to place an ostomy.[
CONCLUSION
Sacral tumors continue to pose a challenge in the field of spinal surgery, as their slow growth and relative clinical silence over prolonged periods promote the onset of debilitating symptoms once clinically manifested. In this review, sacral tumors were identified based on unique clinical presentations and markers of diagnosis. Surgical approaches for the resection of sacral tumors were described as well as ideal postoperative management to mitigate long-term sequelae and tumor recurrence.
To quickly identify the onset of tumor formation in the sacrum, it is imperative patients engage in regular screenings for highly metastatic cancers including those of the lung, prostate, and breast which are commonly found to metastasize to bone. Because metastasis to bone accounts for almost half of all sacral tumor cases, regular screening allows the physician ample opportunity to utilize diagnostic imaging to investigate an oncological etiology of a patient’s localized chief complaint, particularly from individuals with a prior history of any of the aforementioned primary cancerous lesions. Research and development into genetic markers of individual tumors would aid in rapid detection that can be missed by diagnostic imaging in the early stages of tumor formation. Unfortunately, markers for sacral tumors are rare and in many cases nonspecific. Until primary tumor markers of high sensitivity and specificity are shown to be clinically viable, imaging will remain the most effective diagnostic tool currently available. As such, caution must be placed with chief complaints of low back pain or radiculopathy and should include sacral tumors in the differential diagnosis with appropriate follow-up.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK.editors. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more personalized approach to cancer staging. CA Cancer J Clin. 2017. 67: 93-9
2. Angelini A, Ruggieri P. A new surgical technique (modified Osaka technique) of sacral resection by posterior-only approach: Description and preliminary results. Spine (Phila Pa 1976). 2013. 38: E185-92
3. Benzel EC, Steinmetz MP.editors. Benzel’s Spine Surgery: Techniques, Complication Avoidance, and Management. Philadelphia, PA: Elsevier; 2017. p.
4. Bydon M, De la Garza-Ramos R, Gokaslan ZL.editors. Editorial: Total sacrectomy for malignant sacral tumors via a posterior-only approach. J Neurosurg Spine. 2015. 22: 561-2
5. Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World J Orthop. 2016. 7: 109-16
6. Clarke MJ, Dasenbrock H, Bydon A, Sciubba DM, McGirt MJ, Hsieh PC. Posterior-only approach for en bloc sacrectomy: Clinical outcomes in 36 consecutive patients. Neurosurgery. 2012. 71: 357-64
7. David OI, Lupaşcu-Ursulescu CV, Lupaşcu CD, Sandu AM, Strâmbu VD, Cristian DA. Histopathological diagnosis and its correlations with anatomoclinical features, surgical approach and postoperative prognosis in sacral tumors. Rom J Morphol Embryol. 2017. 58: 393-408
8. Deutsch H, Mummaneni PV, Haid RW, Rodts GE, Ondra SL. Benign sacral tumors. Neurosurg Focus. 2003. 15: E14
9. Diel J, Ortiz O, Losada RA, Price DB, Hayt MW, Katz DS. The sacrum: Pathologic spectrum, multimodality imaging, and subspecialty approach. Radiographics. 2001. 21: 83-104
10. Ebraheim NA, Thomas BJ, Fu FH, Muller B, Vyas D, Niesen M, Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Kao LS, Hunter JG.editors. Orthopedic surgery. Schwartz’s Principles of Surgery. New York: McGraw-Hill Education; 2019. p.
11. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980. 153: 106-20
12. Feldenzer JA, McGauley JL, McGillicuddy JE. Sacral and presacral tumors: Problems in diagnosis and management. Neurosurgery. 1989. 25: 884-91
13. Fletcher CD.editors. World Health Organization. WHO Classification of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2013. p.
14. Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL. En bloc resection of primary sacral tumors: Classification of surgical approaches and outcome. J Neurosurg Spine. 2005. 3: 111-22
15. Francis GJ, Ngo-Huang A, Rhines LD, Bruera E. The challenges of providing rehabilitation for patients undergoing sacrectomy: Two case reports. Eur J Phys Rehabil Med. 2019. 55: 526-9
16. Fritchie KJ, John I, Reisner HM.editors. Soft tissue and bone pathology. Pathology: A Modern Case Study. New York: McGraw-Hill Education; 2020. p.
17. Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005. 87: 2211-6
18. Gao S, Zheng Y, Liu X, Tian Z, Zhao Y. Effect of early fasting and total parenteral nutrition support on the healing of incision and nutritional status in patients after sacrectomy. Orthop Traumatol Surg Res. 2018. 104: 539-44
19. Guo Y, Yadav R. Improving function after total sacrectomy by using a lumbar-sacral corset. Am J Phys Med Rehabil. 2002. 81: 72-6
20. Hugate RR, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: When is reconstruction necessary?. Clin Orthop Relat Res. 2006. 450: 82-8
21. Kemp WL, Burns DK, Brown TG.editors. Pathology of the Bones and Joints. Pathology: The Big Picture. New York: The McGraw-Hill Companies; 2008. 19:
22. Kiatisevi P, Piyaskulkaew C, Kunakornsawat S, Sukunthanak B. What are the functional outcomes after total sacrectomy without spinopelvic reconstruction?. Clin Orthop Relat Res. 2017. 475: 643-55
23. Kwaan MR, Stewart DB Sr, Dunn KB, Colon rectum, anus. Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Kao LS, Hunter JG.editors. Schwartz’s Principles of Surgery. New York: McGraw-Hill Education; 2019. p.
24. Lam CH, Nagib MG. Nonteratomatous tumors in the pediatric sacral region. Spine (Phila Pa 1976). 2002. 27: E284-7
25. Malelak EB, Lauren C, Argie D, Nugraheni T. Congenital midline spinal hamartoma in a 5-month-old infant. World Neurosurg. 2020. 145: 142-7
26. Manaster BJ, Graham T. Imaging of sacral tumors. Neurosurg Focus. 2003. 15: E2
27. Mavrogenis AF, Patapis P, Kostopanagiotou G, Papagelopoulos PJ. Tumors of the sacrum. Orthopedics. 2009. 32: 342-56
28. Mhatre P, Hudgins PA, Hunter S. Dermoid cyst in the lumbosacral region: Radiographic findings. AJR Am J Roentgenol. 2000. 174: 874-5
29. Minasian T, Claus C, Hariri OR, Piao Z, Quadri SA, Yuhan R. Chondromyxoid fibroma of the sacrum: A case report and literature review. Surg Neurol Int. 2016. 7: S370-4
30. Patel N, Maturen KE, Kaza RK, Gandikota G, Al-Hawary MM, Wasnik AP. Imaging of presacral masses-a multidisciplinary approach. Br J Radiol. 2016. 89: 20150698
31. Patel SR, Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J.editors. Soft tissue and bone sarcomas and bone metastases. Harrison’s Principles of Internal Medicine. New York: McGraw-Hill Education; 2018. p.
32. Payer M. Neurological manifestation of sacral tumors. Neurosurg Focus. 2003. 15: E1
33. Peh WC, Koh WL, Kwek JW, Htoo MM, Tan PH. Imaging of painful solitary lesions of the sacrum. Australas Radiol. 2007. 51: 507-15
34. Puffer RC, Gates MJ, Copeland W, Krauss WE, Fogelson J. Tarlov cyst causing sacral insufficiency fracture. Oper Neurosurg (Hagerstown). 2017. 13: E4-7
35. Sahakitrungruang C, Chantra K, Dusitanond N, Atittharnsakul P, Rojanasakul A. Sacrectomy for primary sacral tumors. Dis Colon Rectum. 2009. 52: 913-8
36. Stephens M, Gunasekaran A, Elswick C, Laryea JA, Pait TG, Kazemi N. Neurosurgical management of sacral tumors: Review of the literature and operative nuances. World Neurosurg. 2018. 116: 362-9
37. Varga PP, Szövérfi Z, Lazary A. Surgical treatment of primary malignant tumors of the sacrum. Neurol Res. 2014. 36: 577-87
38. Wang Y, Liang W, Qu S, Zhang Y, Du Z, Ji T. Assessment of patient experiences following total sacrectomy for primary malignant sacral tumors: A qualitative study. World J Orthop. 2019. 120: 1497-504
39. Zang J, Guo W, Yang R, Tang X, Li D. Is total en bloc sacrectomy using a posterior-only approach feasible and safe for patients with malignant sacral tumors?. J Neurosurg Spine. 2015. 22: 563-70
40. Zhu R, Cheng LM, Yu Y, Zander T, Chen B, Rohlmann A. Comparison of four reconstruction methods after total sacrectomy: A finite element study. Clin Biomech (Bristol Avon). 2012. 27: 771-6