- Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- Department of Anaesthesiology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia
- Collaborative Microelectronic Design Excellence Center (CEDEC), Universiti Sains Malaysia, Bayan Lepas, Malaysia
- School of Computer Science, University of Nottingham Malaysia, Semenyih, Malaysia
- Centre for Intelligent Signal and Imaging Research, Universiti Teknologi PETRONAS, Bandar Seri Iskandar, Malaysia.
Correspondence Address:
Zamzuri Idris, Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia.
DOI:10.25259/SNI_118_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zamzuri Idris1, Ang Song Yee1, Wan Mohd Nazaruddin Wan Hassan2, Mohamad Hasyizan Hassan2, Laila Ab Mukmin2, Khairu Anuar Mohamed Zain3, Asrulnizam Abd Manaf3, Rodney Petrus Balandong4, Tong Boon Tang5. Clinical outcomes and thermodynamics aspect of direct brain cooling in severe head injury. 28-Apr-2023;14:158
How to cite this URL: Zamzuri Idris1, Ang Song Yee1, Wan Mohd Nazaruddin Wan Hassan2, Mohamad Hasyizan Hassan2, Laila Ab Mukmin2, Khairu Anuar Mohamed Zain3, Asrulnizam Abd Manaf3, Rodney Petrus Balandong4, Tong Boon Tang5. Clinical outcomes and thermodynamics aspect of direct brain cooling in severe head injury. 28-Apr-2023;14:158. Available from: https://surgicalneurologyint.com/surgicalint-articles/12293/
Abstract
Background: Brain cooling therapy is one of the subjects of interest, and currently, data on direct brain cooling are lacking. Hence, the objective is to investigate the clinical outcomes and discuss the thermodynamics aspect of direct brain cooling on severely injured brain patients.
Methods: This pilot study recruited the severely injured brain patients who were then randomized to either a direct brain cooling therapy group using a constant cooling temperature system or a control group. All studied patients must be subjected to an emergency neurosurgical procedure of decompressive craniectomy and were monitored with intracranial pressure, brain oxygenation, and temperature. Further, comparison was made with our historical group of patients who had direct brain cooling therapy through the old technique.
Results: The results disclosed the direct brain cooling treated patients through a newer technique obtained a better Extended Glasgow Outcome Score than a control group (P P
Conclusion: Direct brain cooling is feasible, safe, and affects the clinical outcomes of the severely traumatized brain, and physics of thermodynamics may play a role in its pathophysiology.
Keywords: Brain cooling, Brain temperature, Brain thermodynamics, Brainwaves, Decompressive craniectomy
INTRODUCTION
The pathophysiology of traumatic brain injury can be divided into primary and secondary injuries. The secondary injuries are related to increased cell death and poor neurological outcomes.[
MATERIALS AND METHODS
A newly innovated direct brain cooling therapy system was constructed by a local group of engineers and neurosurgeons using the system on chip-based and microelectronic mechanical system-based sensors. This direct brain cooling system was registered and published as D-brain cooling machine.[
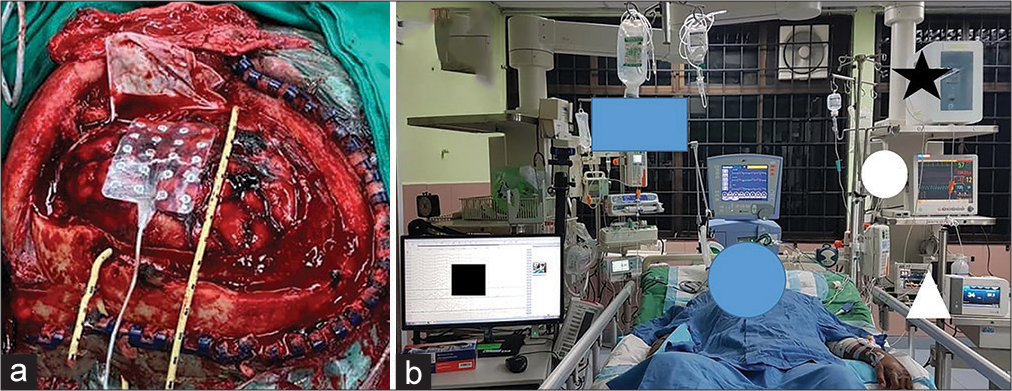
Figure 1:
The direct brain cooling clinical study. (a) A severely head-injured patient was treated with decompressive craniectomy, removal of any surgical lesions, and duraplasty. The image also shows a grid electrode and a coolant catheter which were laid onto the cortical brain surface. (b) A studied patient received a direct brain hypothermia therapy (black star) and was monitored with intracranial pressure (round white circle), brain oxygenation and temperature (white triangle), and electrocorticographic brainwaves (black square).
With regard to the neurosurgical procedure, a standard decompressive craniectomy (DC) of either unilateral or bifrontal was used. The unilateral DC was used for patients with significant unilateral surgical lesion such as subdural or contusional hemorrhage with midline shift to the opposite side and effacement of basal cisterns. For the bifrontal DC, it was used whenever there was a significant surgical lesion such as contusions at the frontal lobe(s) or the presence of brain swelling with effacement of the basal cisterns without a midline shift. Our unilateral neurosurgical DC was a standard operation covering the frontal, parietal, and temporal lobes. Besides bony decompression, duraplasty with pericranium was made after the removal of any surgical lesions. At the end of the surgery, the ICP probe was inserted through a burr hole into the ventricle or parenchyma of the opposite hemisphere. For the Licox probe, it was inserted into the ipsilateral side of the DC hemisphere. The Licox catheter which measured the brain oxygenation and temperature laid superficially at the subcortical region of the brain (>5 mm in depth). The abnormally looking brain area was selected during the surgery for its probe insertion. Finally, the ECoG grid of either 5 × 4 or 8 × 4 electrodes to monitor the brainwaves was laid onto the surface of the decompressed brain. The ECoG brainwave monitoring was completed using the NicoletOne system with adjustable sensitivity format ranging from 10 to 5000 µV/cm, low and high cuts of 1 and 50 Hz and time-based of 30 mm/s. The brainwaves’ power spectrum (or energy per unit of time) (µV2/Hz) was automatically calculated by the quantitative ECoG power band software in the NicoletOne system. For the specific alpha connectivity (coherence) and alpha power analysis, an ECOG preprocessing pipeline was used with bandpass filtering within 1–43 Hz. The excessive amplitude of filtered signal or slower frequency activity was automatically marked and excluded from analysis using the auto-reject library.
The therapies after the DC surgery were the standard ones for patients with severe head injuries – these include sedation (propofol, 10 mg/h, and fentanyl, 50 mcg/h) without muscle paralysis agent; ventilation; CSF drainage; mannitol or hypertonic saline; and finally, thiopentone coma therapy for those with persistent increase or refractory ICP of >20 mmHg. Regarding direct focal brain cooling, it was started in neurointensive care by continually irrigating the brain with cold Hartmann’s solution using our newly innovated machine. The temperature of the infused fluid was made constant at 32°C throughout the treatment period. Due to the patient’s head position, a second larger drained tube was inserted at the lower part of the craniectomy flap and outside the dura which was loosely closed to drain the excess fluid with a low suction pressure. For the control group, no cooling therapy was given. Nevertheless, all controlled patients were also monitored with the ICP, Licox, and ECoG grid. The assessment of the outcomes was conducted through a dichotomized Extended Glasgow Outcome Scale (GOSE) at discharge and 6 months after the trauma as (1) good neurological outcome group (GOSE 4–8) and (2) poor neurological outcome group (GOSE 1–3). The statistical analysis was completed using the Statistical Package for the Social Sciences (SPSS; IBM, Chicago, IL), version 26.0 to mainly compare the constant cooling temperature at 32°C group or group A with the non-cooling, control, or group B. In addition, we also included and compared the current data with our historical brain cooling data that consisted of a group of the first 15 recruited-patients who had direct brain cooling therapy through the old method. The whole data of this study was published in 2014.[
RESULTS
The results were divided into two parts. Part one comprised the demographic, clinical parameters, and outcomes data for the three groups whereas part two contained case examples for cooling (group A) and non-cooling (group B) patients with their related brainwave morphology, energy shift, alpha wave connectivity or coherence, and power spectrum for the alpha wave.
Clinical parameters and outcomes
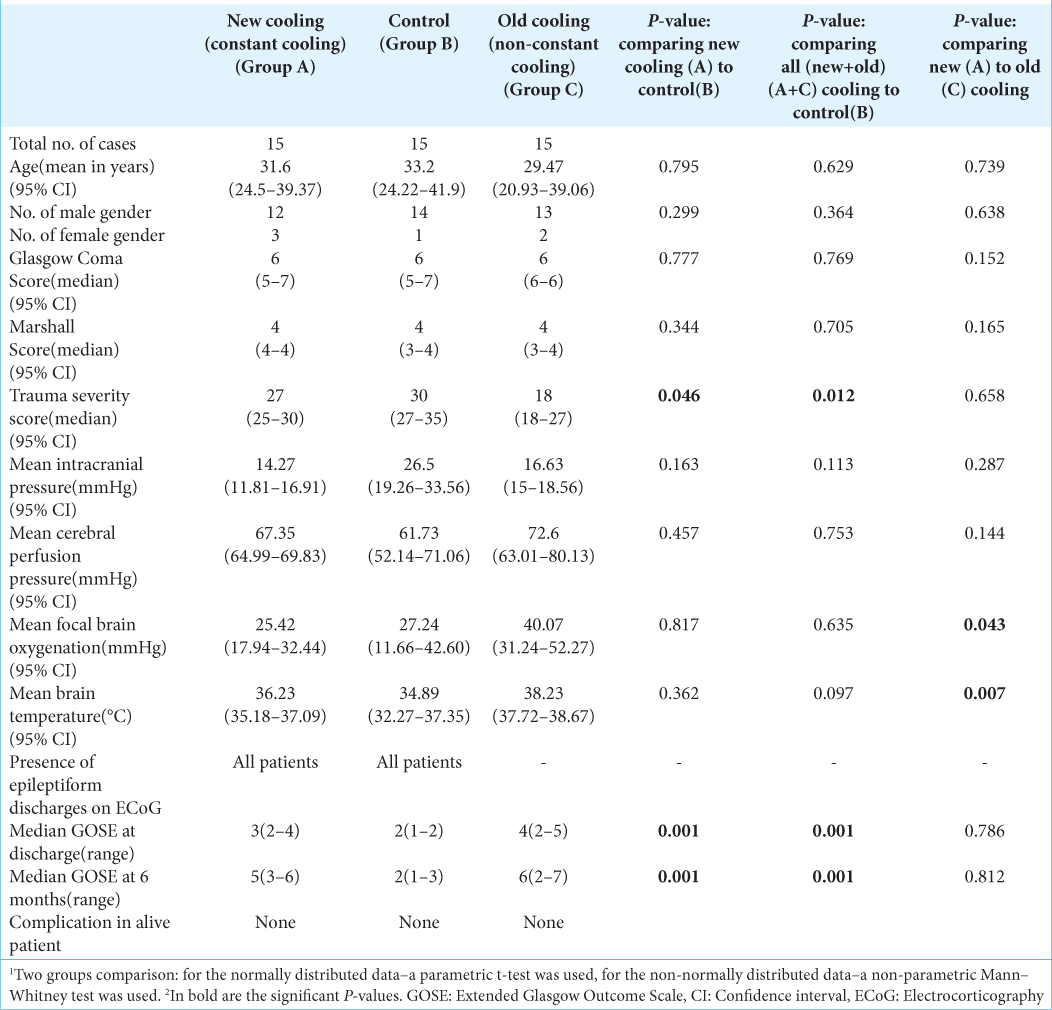
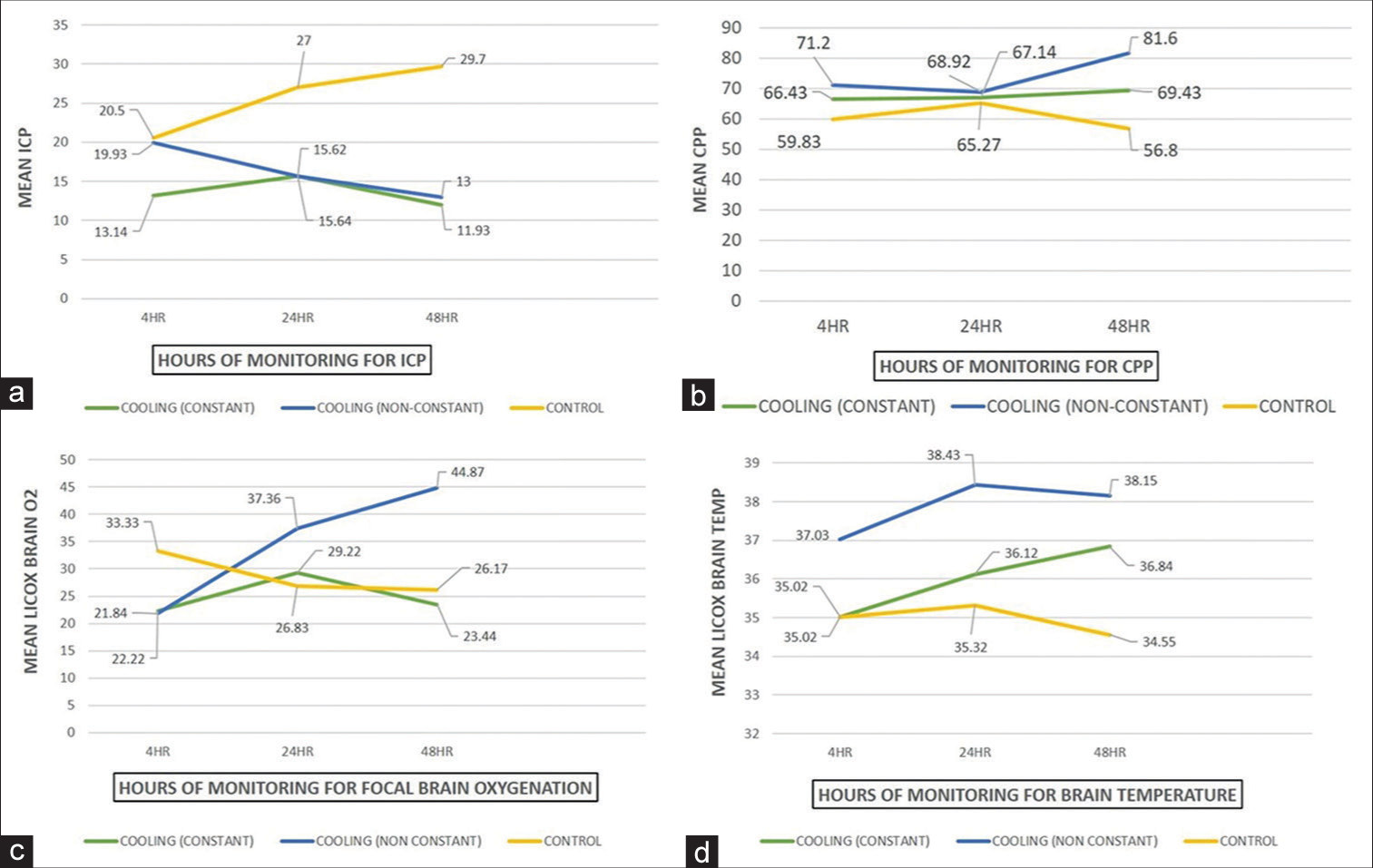
There were three groups analyses: constant cooling (new/ group A), control (group B), and non-constant cooling group (old/group C) [
Concerning the temporal pattern of the monitored clinical parameters [
Case examples: Brainwave morphology and energy in cooling and non-cooling patients
A case of direct brain cooling
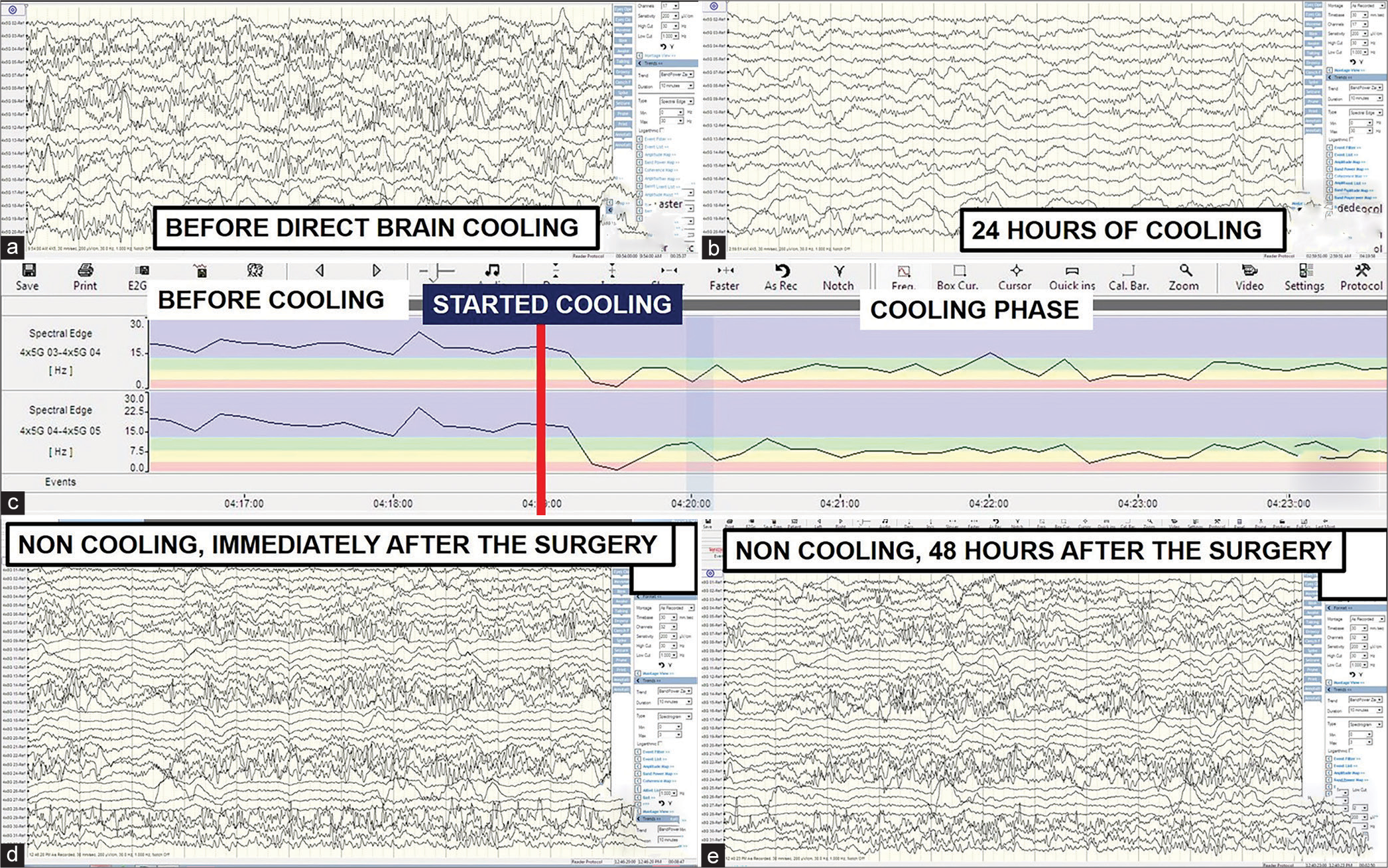
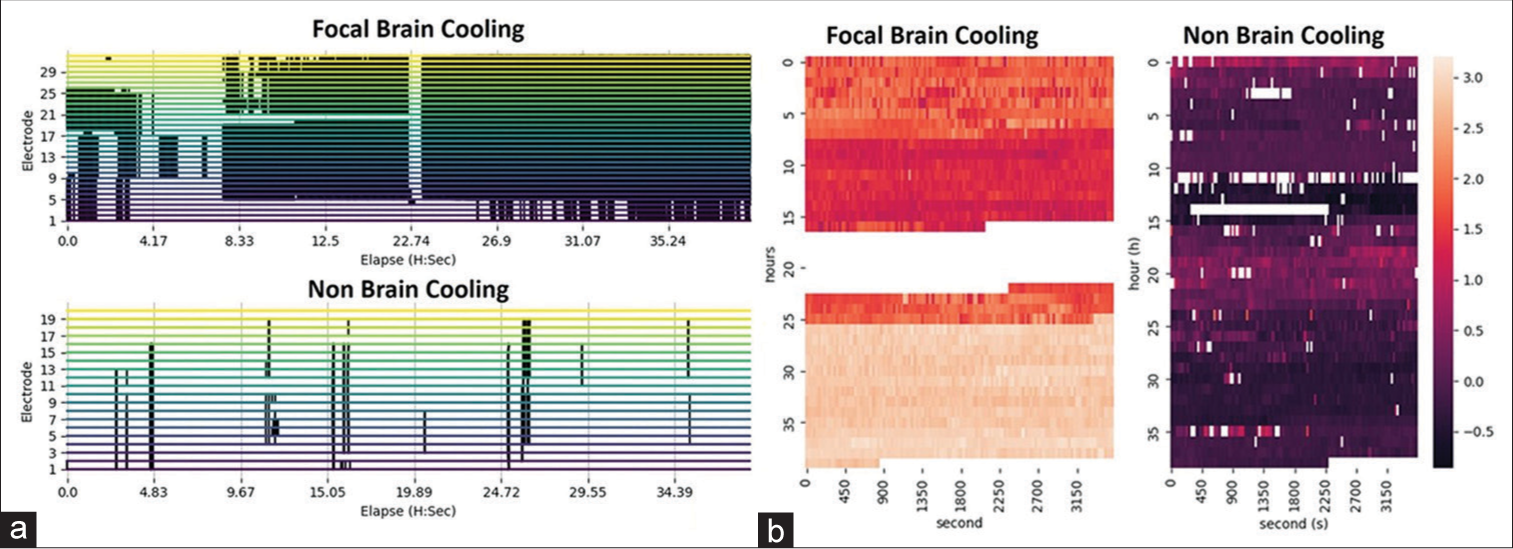
A 30-year-old lady involved in a road traffic accident with GCS of 7 was noted to have a 1.5 cm thickness of the right frontotemporoparietal acute subdural hemorrhage (SDH), a midline shift to the opposite side of 1 cm, bifrontal contusions, traumatic subarachnoid hemorrhage (tSAH), and brain swelling with effacement of basal cisterns. Emergency DC, removal of SDH, laying of a 5 × 4 subdural grid ECoG electrode, ICP, Licox, cooling catheter insertion, and duraplasty were made. Direct brain cooling therapy at 32°C was started soon after the surgery in our neurotrauma intensive care unit (ICU). The recorded brainwave data showed the presence of persistent non-periodic abnormal epileptic activities [
A non-cooling case
A 31-year-old man with a GCS of 6 was admitted following a road traffic accident. His urgent computed tomography brain disclosed the presence of an acute right temporal epidural hemorrhage of 2 cm in thickness, an acute right SDH of 1 cm in thickness, and an opposite acute thin SDH with contusions covering the frontal, temporal and parietal areas, tSAH, and brain swelling. He underwent emergency hemispheric DC, removal of blood clots, duraplasty, Licox, subdural grid, and ICP monitoring. His direct cortical brainwave recording revealed a persistent presence of subclinical and abnormal epileptiform discharges [
Figure 3:
Brainwave morphology, epileptic activity, and energy. (a and b) A significant improvement in brainwave morphology without abnormal epileptic activities for the direct brain cooling patient. (c) Direct brain cooling of the same patient at electrodes 3–5 (contused brain) lowers the brainwave spectrum from more beta- (high energy) to delta-alpha range (low energy) with more spectral fluctuation pattern observed after the cooling. (d and e) A persistent presence of subclinical epileptic activities for the non-cooling patient.
DISCUSSION
Therapeutic hypothermia and clinical outcomes
Tier one through tier three are used in managing the severely injured brains with established intracranial hypertension. DC and induced mild hypothermia (32–35°C) are stated under tier three of the management. They are used after failed tier one and two which consist of sedation, analgesia, CSF drainage, mannitol, hypertonic saline, neuromuscular blockade, mild hypocapnia, and CPP management based on autoregulation assessment.[
Besides the commonly monitored clinical parameters, the added values of our study are related to brainwave monitoring using the gold standard subdural grid of ECoG. The direct cortical brainwave study revealed the presence of subclinical seizures in all studied patients which were mitigated or abolished by the cooling therapy. Our case examples depicted improved brainwave morphology after cooling therapy. Detailed analysis revealed lower spectral energy, improved alpha connectivity or coherence, and alpha power in the cooling treated patients. With these findings, we recommended routine ECoG monitoring for the severely injured brain who underwent either primary or secondary DC. Concerning direct brain cooling therapy, since only few reported clinical studies are available in current literature, obviously more studies are needed to ascertain its true benefits.[
Brain energy and brain thermodynamics related to DC and direct brain cooling
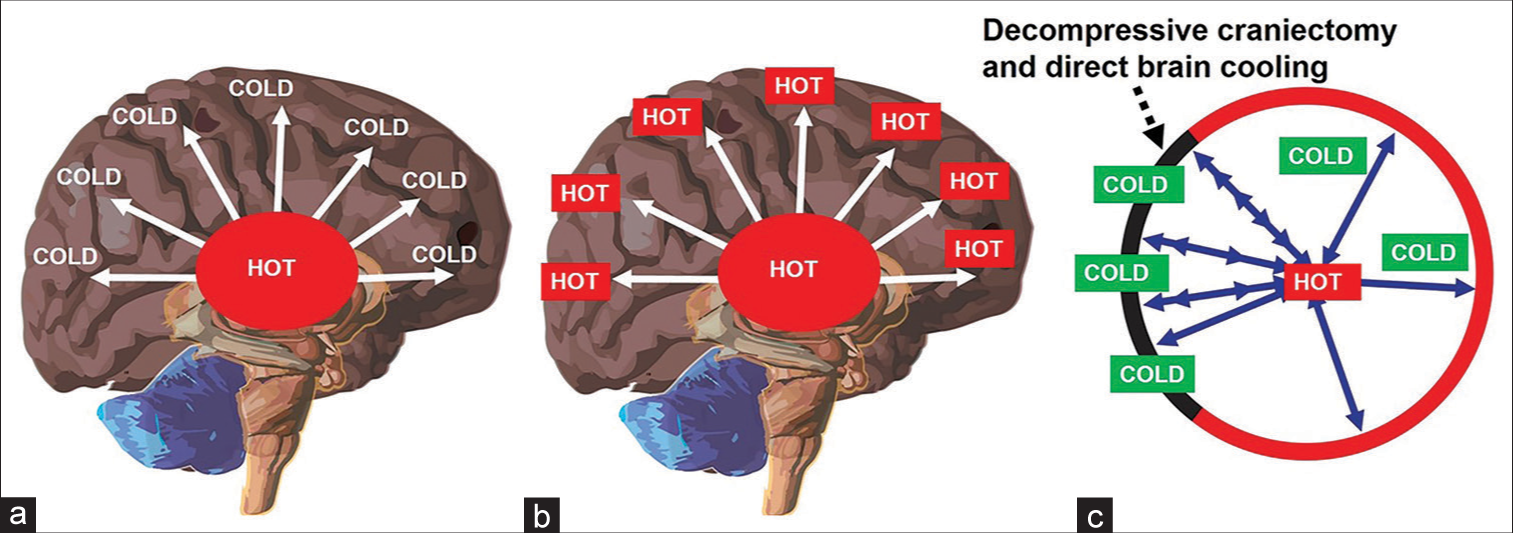
Brain metabolism increases in brain injury. The secondary brain injury is associated with microscopic events that increase metabolism which produces energy and heat.[
Figure 5:
Decompressive craniectomy (DC) and thermodynamics aspect of brain cooling. (a) Presence of temperature gradient inside a healthy person (core is higher than periphery). (b) Loss of temperature gradient in the severely injured brain. (c) Reestablishment of temperature (heat) gradient after synergistic therapy – DC and direct brain cooling (note: cooling effect may spread to the whole brain through cerebrospinal fluid pulsation).
Limitation and recommendation
This study has limitations in term of the sample size and deep nuclei brain temperatures were not studied. Thus, the temperature gradient was not truly established. Hence, future studies should enrol more patients and consider measuring the deep brain region temperature in the severely head-injured patients.
CONCLUSION
In a severely injured brain with compromised cerebral blood perfusion, CSF flow and airways, a rise in cerebral metabolism to accommodate demand may end up with a further rise in ICP and brain heat. To reverse the situation, our synergistic approach using a combined DC and direct brain cooling is proven beneficial with near-normal recorded ICP, CPP, brain oxygenation, and temperature.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Research University (RUi) Grant, No: 1001/PPSP/8012261.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006. 104: 469-79
2. Abouhashem S, Eldawoody H. Functional outcome after primary decompressive craniectomy for acute subdural hematoma in severe traumatic brain injury. Turk Neurosurg. 2022. 32: 211-20
3. Flynn LM, Rhodes J, Andrews PJ. Therapeutic hypothermia reduces intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury: Preliminary data from the Eurotherm3235 trial. Ther Hypothermia Temp Manag. 2015. 5: 143-51
4. Gallup AC, Hack GD. Human paranasal sinuses and selective brain cooling: A ventilation system activated by yawning?. Med Hypotheses. 2011. 77: 970-3
5. Goodman JC, Valadka AB, Gopinath SP, Uzura M, Robertson CS. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit Care Med. 1999. 27: 1965-73
6. Goss JR, Styren SD, Miller PD, Kochanek PM, Palmer AM, Marion DW. Hypothermia attenuates the normal increase in interleukin 1 beta RNA and nerve growth factor following traumatic brain injury in the rat. J Neurotrauma. 1995. 12: 159-67
7. Hawryluk GW, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ. A management algorithm for patients with intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019. 45: 1783-94
8. Hayward JN, Baker MA. A comparative study of the role of the cerebral arterial blood in the regulation of brain temperature in five mammals. Brain Res. 1969. 16: 417-40
9. Herbowski L, Gurgul H. Thermodynamic approach to cerebrospinal fluid circulation. J Neurol Res. 2011. 1: 215-8
10. Idris Z, Yee AS, Hassan WM, Hassan MH, Zain KA, Manaf A. A clinical test for a newly developed direct brain cooling system for the injured brain and pattern of cortical brainwaves in cooling, noncooling, and dead brain. Ther Hypothermia Temp Manag. 2022. 12: 103-14
11. Idris Z, Mustapha M, Abdullah JM, Agrawal A, editors. Neurointensive care monitoring for severe traumatic brain injury. Brain Injury-Pathogenesis, Monitoring, Recovery and Management. London: IntechOpen; 2012. p.
12. Idris Z, Zenian MS, Muzaimi M, Hamid WZ. Better glasgow outcome score, cerebral perfusion pressure and focal brain oxygenation in severely traumatized brain following direct regional brain hypothermia therapy: A prospective randomized study. Asian J Neurosurg. 2014. 9: 115-23
13. Isa R, Adnan WA, Ghazali G, Idris Z, Ghani AR, Sayuthi S. Outcome of severe traumatic brain injury: Comparison of three monitoring approaches. Neurosurg Focus. 2003. 15: E1
14. Kiyatkin EA. Brain temperature and its role in physiology and pathophysiology: Lessons from 20 years of thermorecording. Temperature (Austin, Tex). 2019. 6: 271-333
15. Mariak Z, White MD, Lewko J, Lyson T, Piekarski P. Direct cooling of the human brain by heat loss from the upper respiratory tract. J Appl Physiol. 1999. 87: 1609-13
16. Marion DW, Darby J, Yonas H. Acute regional cerebral blood flow changes caused by severe head injuries. J Neurosurg. 1991. 74: 407-14
17. Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997. 336: 540-6
18. Mellergård P. Intracerebral temperature in neurosurgical patients: Intracerebral temperature gradients and relationships to consciousness level. Surg Neurol. 1995. 43: 91-5
19. Mrozek S, Vardon F, Geeraerts T. Brain temperature: Physiology and pathophysiology after brain injury. Anesthesiol Res Pract. 2012. 2012: 989487
20. Nortje J, Menon DK. Traumatic brain injury: Physiology, mechanisms, and outcome. Curr Opin Neurol. 2004. 17: 711-8
21. Nosaka N, Okada A, Tsukahara H. Effects of therapeutic hypothermia for neuroprotection from the viewpoint of redox regulation. Acta Med Okayama. 2017. 71: 1-9
22. Oh JY, Jo K, Joo W, Yoo DS, Park H. Temperature difference between brain and axilla according to body temperature in the patient with brain injury. Korean J Neurotrauma. 2020. 16: 147-56
23. Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet (London, England). 2008. 371: 1955-69
24. Rossi S, Zanier ER, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001. 71: 448-54
25. Stone JG, Goodman RR, Baker KZ, Baker CJ, Solomon RA. Direct intraoperative measurement of human brain temperature. Neurosurgery. 1997. 41: 20-4
26. Szczygielski J, Müller A, Mautes AE, Sippl C, Glameanu C, Schwerdtfeger K. Selective brain hypothermia mitigates brain damage and improves neurological outcome after post-traumatic decompressive craniectomy in mice. J Neurotrauma. 2017. 34: 1623-35
27. Tang Z, Yang K, Zhong M, Yang R, Zhang J, Jiang Q. Predictors of 30-day mortality in traumatic brain-injured patients after primary decompressive craniectomy. World Neurosurgery. 2020. 134: e298-305
28. Wang H, Wang B, Normoyle KP, Jackson K, Spitler K, Sharrock MF. Brain temperature and its fundamental properties: A review for clinical neuroscientists. Front Neurosci. 2014. 8: 307
29. Wang J, Wang S, Zhang W, Wang T, Li P, Zhao X. Proteomic profiling of heat acclimation in cerebrospinal fluid of rabbit. J Proteomics. 2016. 144: 113-22
30. Watts ME, Pocock R, Claudianos C. Brain energy and oxygen metabolism: Emerging role in normal function and disease. Front Mol Neurosci. 2018. 11: 216
31. Yan Y, Tang W, Deng Z, Zhong D, Yang G. Cerebral oxygen metabolism and neuroelectrophysiology in a clinical study of severe brain injury and mild hypothermia. J Clin Neurosci. 2010. 17: 196-200