- Department of Internal Medicine, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- Department of Histopathology, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- Department of Endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- Department of Endocrinology and Telemedicine, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- Department of Radiology, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Pinaki Dutta, Department of Endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_1001_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sanish Ancil1, Kirti Gupta2, Subin S3, Liza Das4, Chirag Kamal Ahuja5, Rajesh Chhabra6, Sivashanmugam Dhandapani6, Pinaki Dutta3. Clinico-radio-pathological predictors of outcomes in patients with acromegaly undergoing endoscopic transsphenoidal surgery. 02-Aug-2024;15:268
How to cite this URL: Sanish Ancil1, Kirti Gupta2, Subin S3, Liza Das4, Chirag Kamal Ahuja5, Rajesh Chhabra6, Sivashanmugam Dhandapani6, Pinaki Dutta3. Clinico-radio-pathological predictors of outcomes in patients with acromegaly undergoing endoscopic transsphenoidal surgery. 02-Aug-2024;15:268. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13024
Abstract
Background: Acromegaly is a rare chronic endocrine disorder with variable biochemical remission rates from 40% to 85%. Hence, understanding the factors predicting biochemical cures helps in planning targeted and personalized treatment. We aimed to study the various clinico-radio-pathological predictors of outcomes in patients with pituitary neuroendocrine tumor (PitNET) who underwent transsphenoidal surgery (TSS) at 3 months follow-up.
Methods: Our cohort included 61 consecutive patients with acromegaly treated at an institute in northwest India between January 2019 and June 2021. The outcomes of TSS were assessed at the end of 3 months postoperatively as defined by Endocrine Society Guidelines 2014.
Results: The mean age at diagnosis was 38 ± 12 years, with the majority being females (67.2%). The median tumor volume was 2376 mm3 with high insulin-like growth factor-1 levels (3.12 ± 1.76 times the upper reference limit). Forty-two patients (68.8%) had radiological evidence of cavernous sinus invasion. Overall, the biochemical remission rate at 3 months was 34.4%. Unlike preoperative Knosp grading, T2-hypointensity was not predictive of biochemical remission. The granularity of PitNET, as well as immunohistochemical (IHC) markers such as Ki-67 index somatostatin receptor subtype (SSTR2/5) and low-molecular-weight cytokeratin (CAM5.2) expression, failed to show any significant correlation with remission.
Conclusion: Overall, bulky tumors, higher hormone burden, and advanced Knosp grades translated to lower rates of biochemical remission in the present study cohort. Contrary to earlier studies, conventional IHC markers such as Ki-67, SSTR2/5, and CAM5.2 were not useful for predicting biochemical remission at 3 months.
Keywords: Acromegaly, Histopathology, Pituitary neuroendocrine tumor (PitNET), Radiology, Remission, Transsphenoidal surgery
INTRODUCTION
Acromegaly is a chronic endocrine disorder characterized by hypersecretion of growth hormone (GH), most often secondary to a functioning pituitary neuroendocrine tumor (PitNET). Early diagnosis and timely personalized management of acromegaly are required for alleviating morbidity and financial burden.[
MATERIALS AND METHODS
The study cohort consists of 61 patients with acromegaly, diagnosed based on Endocrine Society guideline 2014,[
All patients were reevaluated at 3 months to assess biochemical, clinical, and radiological remission following TSS. The composite clinical remission was defined by the resolution of four clinical features, which include subjective improvement in acral enlargement and a decrease in headache, sweating, and seborrhea, while the complete absence of tumor residue defined radiological remission. We also calculated the global SAGIT score.[
Statistical analysis
The outcome groups were compared using the Chi-square or Fischer exact test. Univariate, followed by multivariate logistic regression, was applied to find significant predictors of remission in TSS. All tests were carried out at a 5% level of significance; that is, P < 0.05 was considered significant. All analyses were carried out using IBM-Statistical Package for the Social Sciences Statistics version 21 software (IBM Corporation, Armonk, New York, USA).
RESULTS
Clinical features
The mean age at diagnosis of patients in the study cohort was 38 ± 12 years with a female-to-male ratio of 2:1. The mean lag period (from first symptom to diagnosis) was 39 ± 33 months. The observed clinical features were as follows (in decreasing order of prevalence): acral enlargement (97%), coarse facial features (97%), sweating (75%), headache (75%), menstrual irregularities in females (36%), arthralgia (21%), obstructive sleep apnea (17%), and self-reported visual symptoms (5%). However, formal visual field assessment (n = 49) revealed field defects in the right eye (n = 18/49: 36.7%) and left eye, respectively (n = 22/49; 44.9%). Among comorbidities, 14 (23%) of the participants had diabetes mellitus, while 20 (33%) had hypertension.
Preoperative hormonal analysis revealed hypogonadism in 40%, hypocortisolism in 21%, concomitant hypothyroidism in 10%, and panhypopituitarism in 5%. Postoperatively, the proportion of patients with subjective resolution of observed clinical features was as follows (in decreasing order): headache (58%), acral enlargement (57%), seborrhea (52%), and sweating (51%). Composite clinical remission defined by resolution of the above four clinical features was 42%. The postoperative remission rates of diabetes and hypertension were 86% and 33%, respectively. The mean global SAGIT score (n = 51) in our study cohort was 13 + 2.6.
Radiological findings
Preoperative
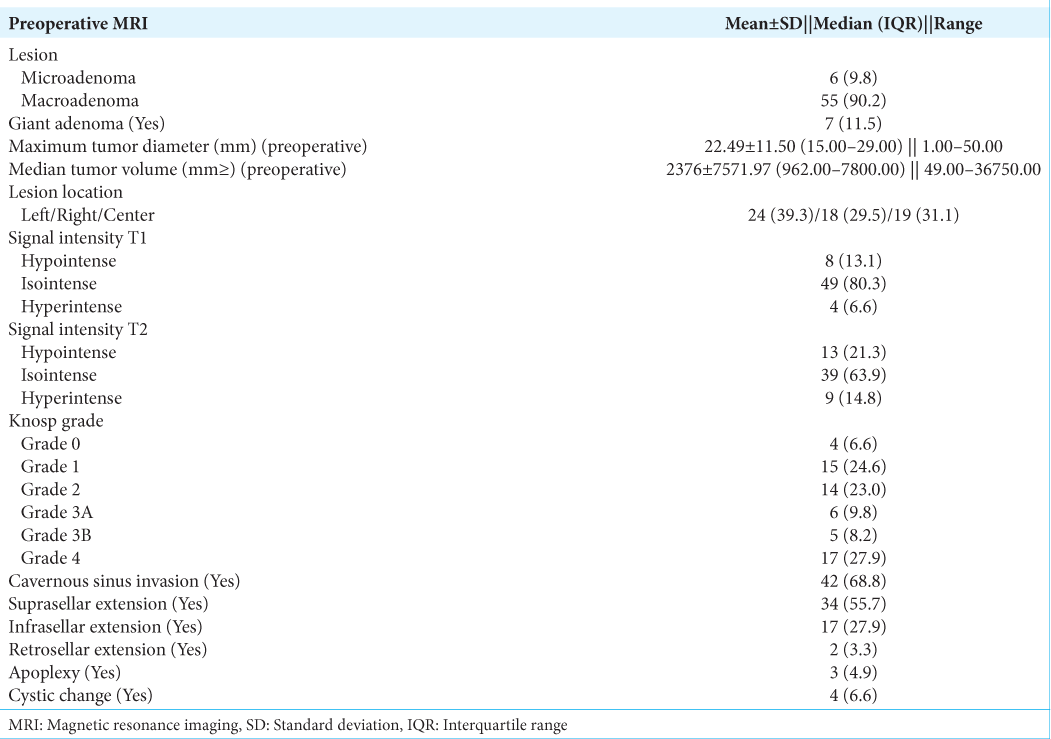
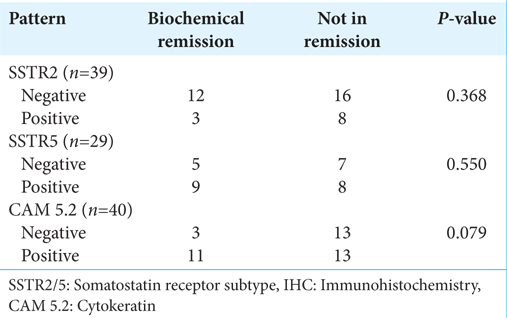
Tumors were classified into microadenoma and macroadenoma based on the maximum tumor diameter in any dimension. The summary of radiological findings is listed in
Postoperative
Even though only 20 patients among 61 subjects (36%) had radiological remission at 3 months, 30 (60%) participants (n = 55; follow-up) had radiological remission on long-term follow-up with a median duration of 49 ± 30 months. The increase in remission rates is due to the cumulative effect of adjuvant therapy. There was no mortality in our study cohort. The relative percentage of patients with cerebrospinal fluid rhinorrhea, carotid artery injury, and postoperative epistaxis was not assessed in the present study, as similar studies have already been done from our center.
Biochemical remission at 3 months
The median preoperative random GH and IGF-1 were 23.22 ng/mL (interquartile range [IQR]: 10.00–46.25) and 750.00 ng/mL (IQR: 499.50–1059.75), respectively. The mean nadir GH following the OGTT (n = 54) was 33.18 ± 43.20 ng/mL, and eight patients had a paradoxical rise of GH following OGTT. Overall, 21 (34.4%) had biochemical remission at 3 months. The remission rates were 83.3% (n = 5/6) and 29% (n = 16/55) among microadenoma and macroadenoma, respectively. Ten subjects had discordant GH- IGF-1 levels, of which five had high GH with low IGF-1, and the remaining five had high IGF-1 with low GH.
Follow-up and adjuvant therapy
We followed up on patients’ serial GH and IGF-1 at regular intervals. Redo surgery was done in 5 (8%) subjects who were not in remission. Patients with persistent disease were subsequently offered various adjuvant therapies. Till the last follow-up data available, 35 (58%) had received adjuvant treatment. Radiotherapy, in the form of either Gamma Knife radiosurgery or intensity-modulated radiotherapy (IMRT), was offered to 52% of the study subjects. The most provided drugs were somatostatin receptor ligands (SRLs) – octreotideLAR (32%), cabergoline (27%), and temozolomide (7%). Thirty-eight (n = 38/53; 72%) study subjects on long-term follow-up achieved biochemical remission with the aid of adjuvant therapy akin to surgery.
Histopathological analysis
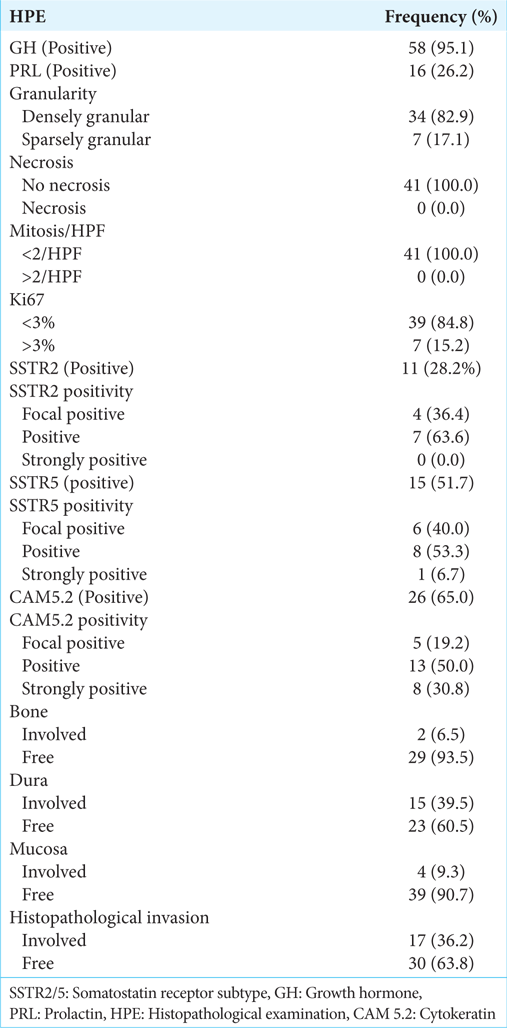
On IHC, 58 specimens (95%) showed GH positivity [
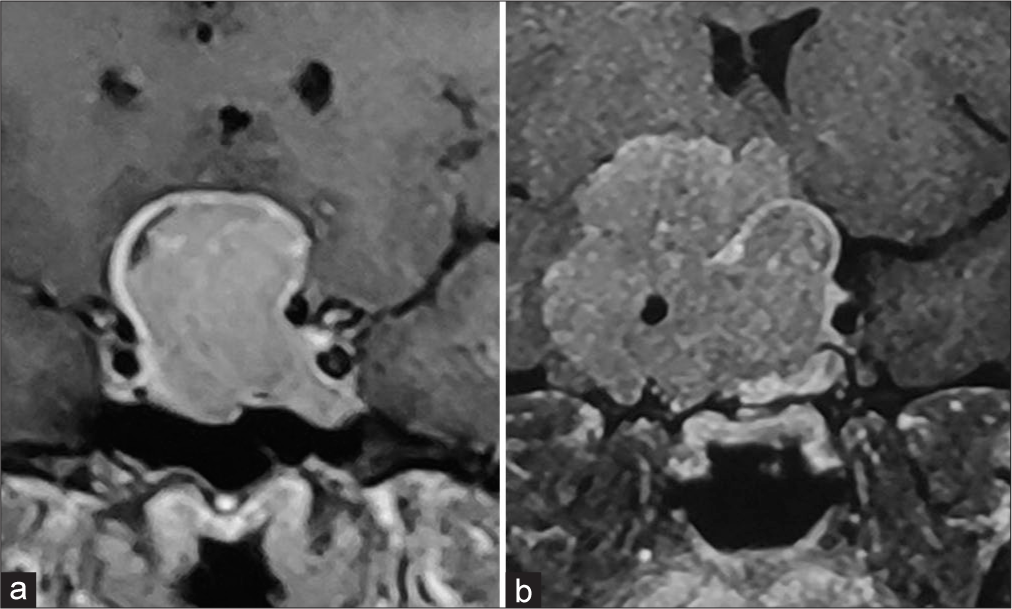
Figure 2:
Densely granulated pituitary neuroendocrine tumor (a) sheets of small round cells with abundant eosinophilic cytoplasm (Hematoxylin and eosin, scale bar 20 μm); (b) strong and diffuse immunoreactivity for growth hormone (GH) (anti-GH, scale bar 20 μm); (c) tumor cells were negative for prolactin, thyroid-stimulating hormone, follicle-stimulating hormone, luteinizing hormone and adrenocorticotropic hormone (immunoperoxidase, scale bar 20 μm); and (d) Ki-67 labeling index is low <3% (immunoperoxidase, scale bar 50 μm).
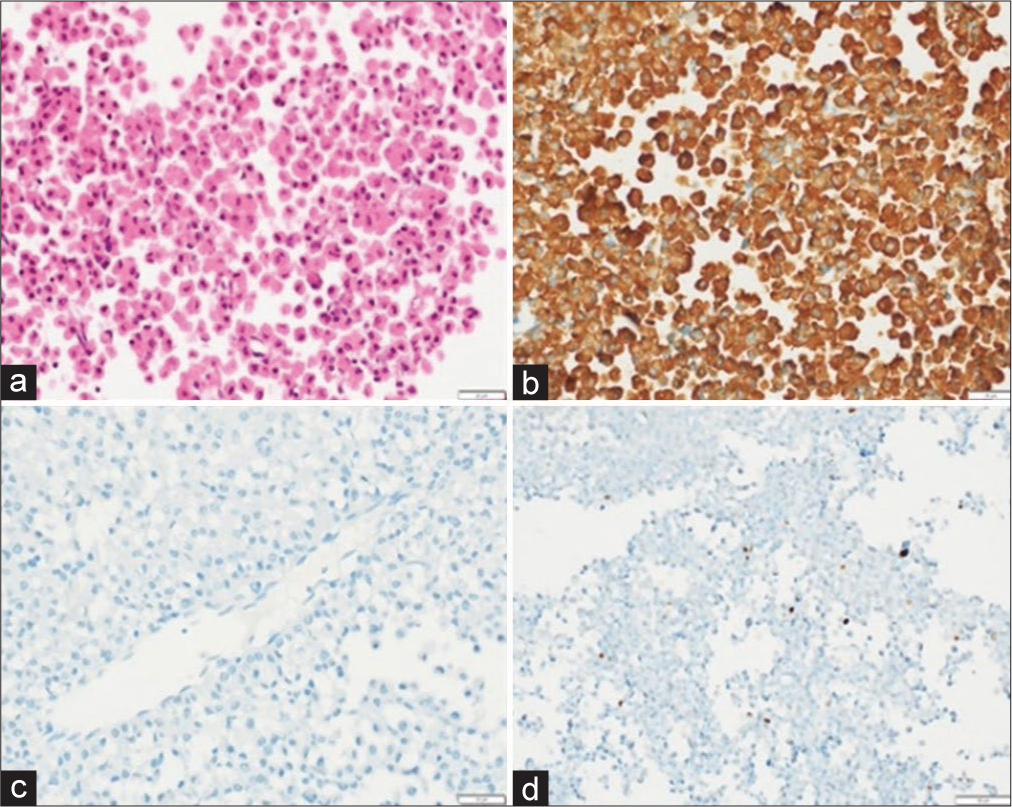
Figure 3:
Sparsely granulated Pituitary neuroendocrine tumor. (a) Low magnification depicting small round cells arranged in sheets interrupted by fine capillaries (Hematoxylin and eosin, scale bar 50 μm); (b) patchy immunoreactivity for growth hormone (GH) (anti-GH, scale bar 20 μm); (c) tumor cells were negative for Prolactin, thyroid-stimulating hormone, follicle-stimulating hormone, luteinizing hormone and adrenocorticotropic hormone (immunoperoxidase, scale bar 20 μm); and (d) Ki-67 labeling index <3% (immunoperoxidase, scale bar 50 μm).
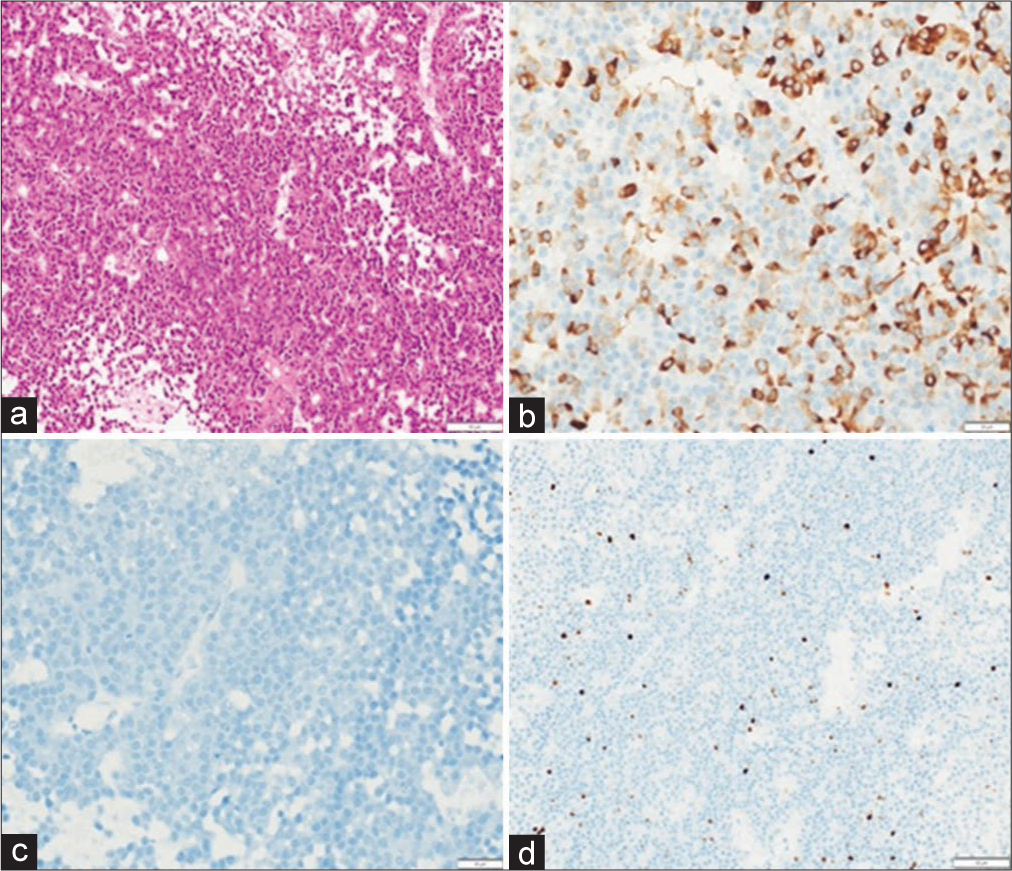
Figure 4:
(a) Pituitary neuroendocrine tumor showing diffuse and strong membranous immunoreactivity for somatostatin receptor subtype (SSTR2) (anti-SSTR5, scale bar 20 μm); (b) focal immunoreactivity for SSTR2a (anti- SSTR2a, scale bar 20 μm); (c) Low-molecular weight cytokeratin (CAM5.2) reveals characteristic fibrous bodies in vast majority of tumor cells (anti-CAM5.2, scale bar 20 μm); and (d) Ki-67 labeling index <3% (immunoperoxidase, scale bar 20 μm).
Figure 5:
(a) Pituitary neuroendocrine tumor showing diffuse and strong membranous immunoreactivity for somatostatin receptor subtype (SSTR5) (anti-SSTR5, scale bar 20 μm); (b) focal and weak immunoreactivity for SSTR 5 (Anti -SSTR 5, scale bar 20 µm), scale bar 20 μm); (c) absence of immunoreactivity with cytokeratin (CAM5.2) (anti-CAM5.2, scale bar 50 μm); and (d) Ki-67 labeling index =5% (immunoperoxidase, scale bar 20 μm).
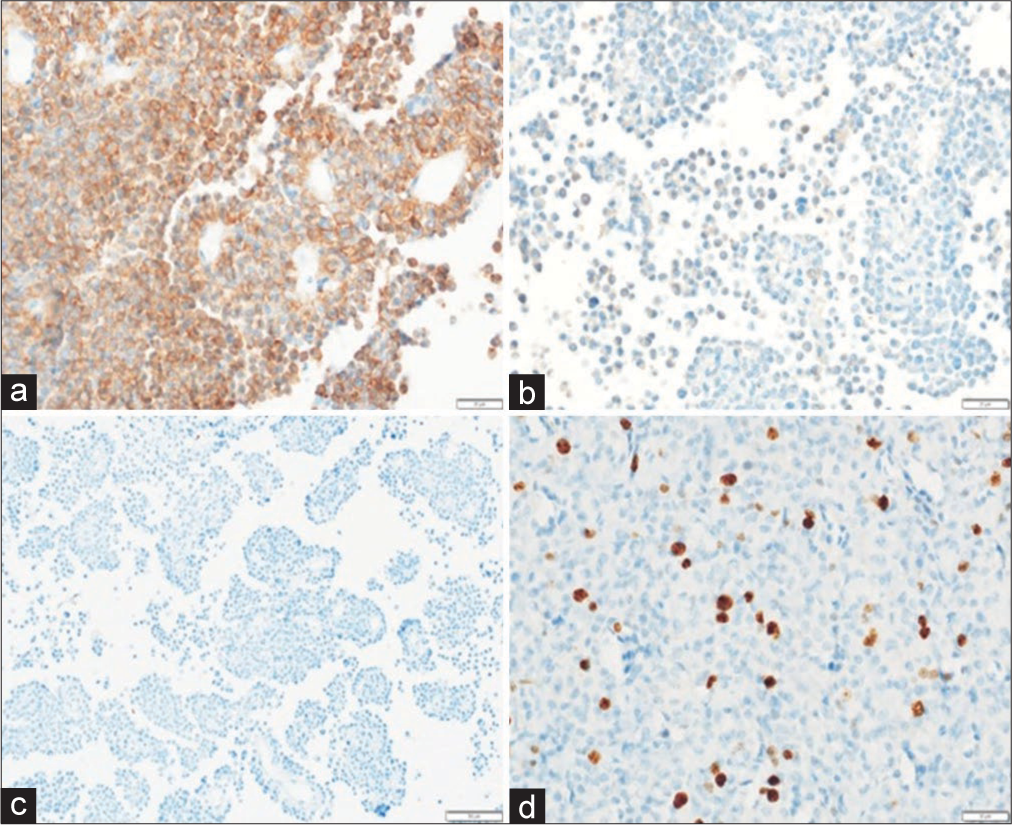
Predictors of biochemical remission
The parameters that had a significant association with biochemical remission at 3 months were younger age at diagnosis, higher tumor diameter and volume, preoperative Knosp grading, radiological evidence of cavernous sinus invasion, composite clinical remission, higher preoperative GH and IGF-1, and percentage change in tumor volume post-TSS. In univariate regression analysis with biochemical remission as dependent variable, variables such as GH nadir (odds ratio [OR] = 0. 93; P = 0.011), age at diagnosis (OR = 1.05, P = 0.050), and preoperative maximum tumor diameter OR = 0.88 (P = 0.004) were found to be significant. Further, logistic regression analysis was done using GH nadir on OGTT, age at diagnosis, and preoperative maximum tumor diameter. On multivariate analysis, GH nadir on OGTT (OR = 0.94, confidence interval [CI] 0.89–1, P = 0.037) and preoperative maximum tumor diameter (OR = 0.90, CI 0.82–0.99, P = 0.029) stood significant [
There was no statistical significance between remission and gender, lag period before diagnosis, comorbidities, or visual acuity. Giant adenoma, T2 signal intensity, and tumor extension other than parasellar invasion did not show statistical significance in predicting outcomes at 3 months. None of the histopathological features, including immunoreactivity for SSTR2, SSTR5, CAM5.2, and Ki67 index, had any significant association in predicting biochemical remission.
DISCUSSION
This is one of the largest single-center studies from Southeast Asia exploring predictors of remission at 3 months following TSS. In this study, bulky tumors, higher hormone burden, and advanced Knosp grades translated to lower rates of biochemical remission. Contrary to earlier studies, conventional IHC markers such as Ki-67, SSTR2/5, and CAM5.2 were not useful for predicting biochemical remission at 3 months.
The present study cohort includes young patients with lesser lag periods and large somatotropinoma with high hormone burden.[
The overall biochemical and radiological remission rates were 34.4% and 36.1%, respectively. The relative remission rates among microadenoma and macroadenoma were 83.3% (n = 5/6) and 29% (n = 16/55), respectively.
Ten subjects had discordant GH- IGF-1 levels, of which five had high GH with low IGF-1, and the remaining five had high IGF-1 with low GH. Such discordance between GH and IGF-1 is not unknown and is usually reported in about one-third of patients. This is said to be due to the disruption of a neural or anatomical network of regulation of GH secretion after surgery. Those with elevated IGF-1, irrespective of GH status, are considered to have active disease. Four patients (4/5) with low IGF-1 but elevated random GH were subjected to GHGTT and were found to be in biochemical remission. Overall, the lower biochemical remission rate at 3 months might be due to the dominance of macroadenoma as well as higher rates of cavernous sinus invasion. The functional status of somatotropinoma, determined by nadir GH post-OGTT and IGF-1 levels, positively correlated with size and parasellar invasion predicted by Knosp grading, which translated to lower biochemical and radiological remission. T2 hypointensity predicts densely granulated somatotropinoma, which has better remission rates and response to SRLs.[
Histologically, Pit-NETs are classified based on the tumor cell lineage, cell of origin, secretory nature, and granularity. Somatotropinomas are histologically characterized by the expression of PIT1, GH immunostaining, alpha-subunit of glycoprotein hormones, and cytokeratin staining.[
Based on the secretory granule pattern, somatotropinomas can be further classified into densely granulated and sparsely granulated PitNET.[
All the sparsely granulated somatotroph tumors showed CAM5.2 positivity and avid SSTR2 expression as compared to densely granulated adenoma, though not meeting statistical significance. A previous study by Brzana et al. showed that sparsely granular tumors had less staining for SSTR2 as compared to densely granular tumors.[
We also followed this patient with periodic random GH and IGF-1. Redo surgery was offered to only 5 (8%) patients who were not in biochemical remission. Redo surgery rates are lower in our study cohort due to the following reasons: (1) lack of acceptance of repeat surgery, (2) as majority of our patients had macroadenoma with parasellar extension; hence, patients mainly opted out for IMRT or SRL, and (3) due to financial burden, our center prefer combined IMRT with SRL therapy as a second line therapy after failed surgery in patients with macroadenoma with parasellar invasion rather than SRL alone. The biochemical remission was sustained in only 29.5% without any adjuvant therapy in the present study cohort. Although mechanistically, SSTR2 expression predicts response to SRLs, we did not observe any association with the said parameters. A significant fraction of our patients did not attain biochemical remission even with adjuvant therapy. This underlines the baseline aggressive and resistant nature of PitNET in our study cohort compared to the Western literature. Hence, the larger study might be required to arrive at a definite conclusion regarding ethnic differences in the tumor nature and heterogeneity in response to SRLs.
Strengths and limitations
The major strength of our study was the single-center study cohort, with all radiological and histopathological analyses done by a single experienced neuroradiologist and pathologist, respectively. The limitations of our study were the small sample size. Due to technical limitations and paucity of tissue for processing, SSTR2, SSTR5, and CAM5.2 staining could not be performed in all the study participants. This could have affected the lower sensitivity of histological parameters for predicting remission. The unavailability of pharmacological agents might have affected the overall long-term remission rate at long-term follow-up with adjuvant therapies.
CONCLUSION
The clinic-radiological predictors of biochemical remission at 3 months were as follows: younger age of presentation, higher tumor diameter and volume, higher preoperative GH and IGF-1 levels, Knosp grade, and radiological evidence of cavernous sinus invasion. T2-hypointensity on MRI and IHC markers such as SSTR2/5 and CAM5.2 failed to reveal a correlation with biochemical remission.
Ethical approval
The research/study approved by the Institutional Review Board at Post Graduate Institute of Medical Education and Research, number NK/5923/MD/456, dated January 30, 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Araujo-Castro M, Pascual-Corrales E, Martínez-Vaello V, Baonza-Saiz G, de Silva JQ, Cancela AA. Predictive model of surgical remission in acromegaly: Age, presurgical GH levels and KNOSP grade as the best predictors of surgical remission. J Endocrinol Invest. 2021. 44: 183-93
2. Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res. 2009. 37: 5-20
3. Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO classification of pituitary tumors. Endocr Pathol. 2022. 33: 6-26
4. Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M. Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: A large single center experience. Pituitary. 2013. 16: 490-8
5. Dutta P, Hajela A, Pathak A, Bhansali A, Radotra BD, Vashishta RK. Clinical profile and outcome of patients with acromegaly according to the 2014 consensus guidelines: Impact of a multi-disciplinary team. Neurol India. 2015. 63: 360-8
6. Gadelha MR, Kasuki L, Lim DS, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: An update. Endocr Rev. 2019. 40: 268-332
7. Giustina A, Bevan JS, Bronstein MD, Casanueva FF, Chanson P, Petersenn S. SAGIT®: Clinician-reported outcome instrument for managing acromegaly in clinical practice--development and results from a pilot study. Pituitary. 2016. 19: 39-49
8. Katznelson L, Laws ER, Melmed S, Molitch ME, Murad MH, Utz A. Acromegaly: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014. 99: 3933-51
9. Klöppel G. Tumour biology and histopathology of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007. 21: 15-31
10. Kontogeorgos G. Classification and pathology of pituitary tumors. Endocrine. 2005. 28: 27-35
11. Mete O, Asa SL. Clinicopathological correlations in pituitary adenomas. Brain Pathol. 2012. 22: 443-53
12. Ozisik H, Yurekli BS, Kutbay NO, Altun I, Ertan Y, Saygılı F. Acromegaly with negative immunostaining for growth hormone on the contrary to silent somatotroph tumor. Int Surg J. 2017. 4: 1506-7
13. Potorac I, Beckers A, Bonneville JF. T2-weighted MRI signal intensity as a predictor of hormonal and tumoral responses to somatostatin receptor ligands in acromegaly: A perspective. Pituitary. 2017. 20: 116-20
14. Reincke M, Petersenn S, Buchfelder M, Gerbert B, Skrobek-Engel G, Franz H. The German Acromegaly Registry: Description of the database and initial results. Exp Clin Endocrinol Diabetes. 2006. 114: 498-505
15. Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007. 156: 203-16
16. Scheithauer BW, Horvath E, Kovacs K, Laws ER, Randall RV, Ryan N. Plurihormonal pituitary adenomas. Semin Diagn Pathol. 1986. 3: 69-82
17. Struja T, Briner L, Meier A, Kutz A, Mundwiler E, Huber A. Diagnostic accuracy of basal cortisol level to predict adrenal insufficiency in cosyntropin testing: Results from an observational cohort study with 804 patients. Endocr Pract. 2017. 23: 949-61
18. Zahr R, Fleseriu M. Updates in diagnosis and treatment of acromegaly. Eur Endocrinol. 2018. 14: 57-61