- John Nasseff Neuroscience Specialty Clinic, Part of Allina Health, 225 North Smith Avenue, Suite 500, St. Paul, MN 55102, USA
Correspondence Address:
Camille Schwarzrock
John Nasseff Neuroscience Specialty Clinic, Part of Allina Health, 225 North Smith Avenue, Suite 500, St. Paul, MN 55102, USA
DOI:10.4103/2152-7806.179228
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Schwarzrock C. Collaboration in the presence of cerebral edema: The complications of steroids. Surg Neurol Int 22-Mar-2016;7:
How to cite this URL: Schwarzrock C. Collaboration in the presence of cerebral edema: The complications of steroids. Surg Neurol Int 22-Mar-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/collaboration-in-the-presence-of-cerebral-edema-the-complications-of-steroids-2/
Abstract
Background:Brain tumor patients often present with neurological changes in the presence of cerebral edema. High-dose dexamethasone is often required for symptom management in brain tumor patients. There are limitations in the foundational research that support the recommendations for appropriate prescribing of dexamethasone. Understanding these limitations can help prescribers and care teams collaborate to better manage this unique patient population as well as identify areas for further research.
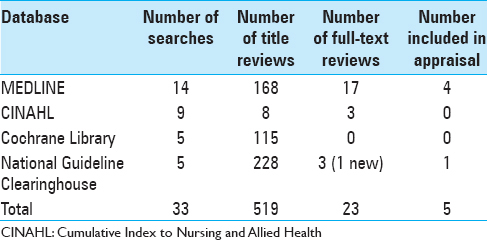
Methods:Evidence-based clinical practice guidelines for the management of adult brain tumor patients were reviewed from several certifying organizations. A complex database search and literature review was completed regarding relevant evidence used within these guidelines and for any supporting literature. The search was limited to MEDLINE, Cumulative Index to Nursing and Allied Health, Cochrane Library, and the National Guideline Clearinghouse using keywords. Each selected evidence-based guideline underwent appraisal using the Johns Hopkins Evidence-based Practice Model.
Results:All clinical practice guidelines identified recommendations for appropriate dosing and tapering of dexamethasone. The management of steroid-induced side effects was addressed in two of the reviewed guidelines. Only one guideline identified specific nursing interventions for monitoring steroid-related side effects. No guideline addressed interval timing of provider or nursing-based interventions as well as the role of collaboration between provider and nurse in monitoring for steroid toxicities.
Conclusions:More high-quality, well-controlled studies are needed around dexamethasone dosing for the management of cerebral edema. Clinical practice guidelines need to encompass both the prescriber and nursing-based interventions. Collaboration between disciplines is a necessity when monitoring and managing steroid-induced toxicities in brain tumor patients. Future evidence-based guidelines need recommendations for appropriate interval screening tests and quantifiable tools needed to aid in monitoring steroid-induced complications.
Keywords: Brain tumor, cerebral edema, dexamethasone, steroids, vasogenic edema
BACKGROUND
Brain tumor patients often present with neurological changes in the presence of cerebral edema. Corticosteroids are a foundational component in the medical management of this patient population.[
PATHOPHYSIOLOGY
Cerebral edema is the result of a disruption in the blood–brain barrier from tumor cells, allowing the accumulation of fluid in the extracellular space. Dexamethasone stabilizes the blood–brain barrier through the regulation of certain mediators found in tumors, ultimately decreasing vascular permeability, and reducing cerebral edema.[
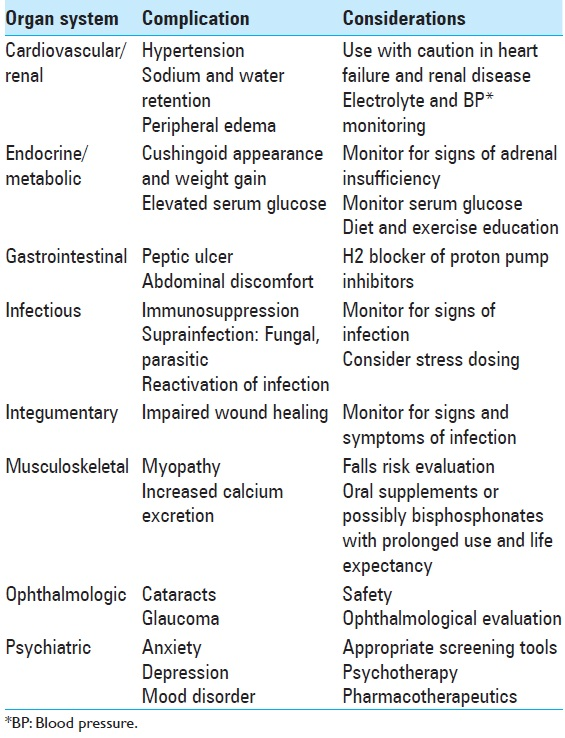
Many dexamethasone-related side effects are due to the inhibition of the hypothalamo-pituitary-adrenal axis and can exhibit effects on virtually every organ system in the body. See
SEARCH STRATEGY
Understanding the underpinnings of evidence-based research is essential when completing successful database searches.[
METHODOLOGY
Clinical practice guidelines are a review of research and nonresearch evidence to systematically develop recommendations that guide patient care. The Johns Hopkins Evidence-based Practice Model was used as a guide to appropriately appraise the evidence of each guideline using a quality rating range from high, good, to low. A high-quality rating has sufficient well-designed studies to support consistent and definitive recommendations. Good quality is based on fairly definitive conclusions with few well-designed studies while acknowledging the evidence limitations. Low quality supports insufficient evidence with inconsistent results.[
RESULTS
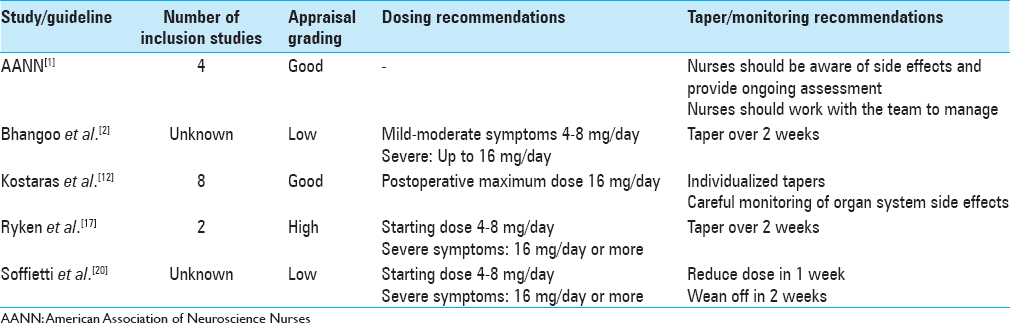
Ryken et al.,[
Two evidence-based practice guidelines were appraised with a good grading. The AANN published guidelines for the adult brain tumor patient. The appraisal quality was good as the evidence was supported by RCTs lacking limitations and included observational studies.[
Finally, two of the five guidelines were given an appraisal grading of low. The European Federation of Neurological Societies published guidelines on the diagnosis and treatment of brain metastases by Soffietti et al.[
DISCUSSION
All clinical practice guidelines agree that appropriate dosing is dependent on the severity of symptoms exhibited by the patient. Steroid use in the asymptomatic patient was difficult to determine based on the limited available evidence. Soffietti et al.[
The RCT by Vecht et al.[
Doses >16 mg/day have not been well researched and careful consideration is warranted in those with severe symptoms associated with increased intracranial pressure.[
One retrospective study analyzed 138 brain tumor patients who received dexamethasone during their radiation treatment.[
Recommendations for monitoring steroid-induced side effects were addressed by two clinical practice guidelines reviewed, Kostaras et al.,[
AANN was the only clinical practice guideline reviewed that addressed specific recommendations regarding appropriate nursing interventions related to corticosteroid potential side effects. AANN recommends that nurses should administer steroids as ordered by the provider and are responsible for monitoring drug-related side effects. Furthermore, nurses should work with their health care team to manage these side effects. AANN made several broad recommendations, including but not limited to monitoring blood glucose levels, hyperglycemia education, assessing for signs of opportunistic infection and muscle weakness, and monitoring for behavioral changes.[
CONCLUSION
Dexamethasone has been used for many decades in the management of cerebral edema in the brain tumor patient. Multiple evidence-based practice guidelines have been published on the appropriate dosing and tapering of dexamethasone to ultimately reduce the risk of steroid toxicities. As discovered, more high-quality, well-controlled, and statistically analyzed research is needed around dexamethasone dosing in the management of cerebral edema in the brain tumor patient. Many of the published guidelines are based on foundational evidence that lack these important qualities.
Furthermore, prevention of steroid-induced complications needs to be encompassed in evidence-based guidelines. Although it is important to know how to treat steroid induce complications, knowing how to screen in order to prevent or limit their toxic effects is crucial. For example, when and how often should fasting plasma glucose be checked? This becomes even more complex in the outpatient setting. Guidelines should also address specific, quantifiable tools that are essential in prevention, such as a baseline depression and/or anxiety screen prior to the initiation of high-dose steroids. Future evidence-based guidelines should provide recommendations for appropriate interval screening tests and quantifiable tools needed to aid in monitoring complications.
Finally, collaboration between disciplines is a necessity when monitoring and managing steroid-induced toxicities in brain tumor patients. Each discipline is being guided by the foundational research of their practice. Care teams need to collaborate to bring the knowledge and evidence that support each discipline. It is essential for evidence-based guidelines to address both nursing and provider interventions. Future guidelines need to be developed in a multidisciplinary fashion bringing the experts from different disciplines together to manage the complex brain tumor patient. Although it is important for nursing to understand and monitor for possible side effects exhibited by their patient, the providers who are prescribing need to collaborate with nursing to manage these complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Last cited on 2015 Jul 18. Available from: http://www.aann.org/pubs/content/guidelines.html .
2. Bhangoo SS, Linskey ME, Kalkanis SN. American Association of Neurologic Surgeons (AANS); Congress of Neurologic Surgeons (CNS). Evidence-based guidelines for the management of brain metastases. Neurosurg Clin N Am. 2011. 22: 97-104
3. Brainin M, Barnes M, Baron JC, Gilhus NE, Hughes R, Selmaj K. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces – Revised recommendations 2004. Eur J Neurol. 2004. 11: 577-81
4. Chalk JB, Ridgeway K, Brophy T, Yelland JD, Eadie MJ. Phenytoin impairs the bioavailability of dexamethasone in neurological and neurosurgical patients. J Neurol Neurosurg Psychiatry. 1984. 47: 1087-90
5. Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005. 44: 61-98
6. Fonkem E, Bricker P, Mungall D, Aceves J, Ebwe E, Tang W. The role of levetiracetam in treatment of seizures in brain tumor patients. Front Neurol. 2013. 4: 153-
7. Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet. 1961. 81: 46-53
8. Greenberg MS.editorsHandbook of Neurosurgery. New York: Thieme; 2010. p.
9. Gupta P, Bhatia V. Corticosteroid physiology and principles of therapy. Indian J Pediatr. 2008. 75: 1039-44
10. Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: Do the benefits outweigh the side-effects?. Support Care Cancer. 2002. 10: 322-8
11. Kim H, Lee JM, Park JS, Jo SA, Kim YO, Kim CW. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: A mechanism of glucocorticoid-induced stabilization of blood-brain barrier. Biochem Biophys Res Commun. 2008. 372: 243-8
12. Kostaras X, Cusano F, Kline GA, Roa W, Easaw J. The Alberta Provincial CNS Tumour Team. Use of dexamethasone in patients with high-grade glioma: A clinical practice guideline. Curr Oncol. 2014. 21: e493-503
13. McCance KL, Huether SE, Brashers VL, Rote NS.editorsPathophysiology the Biologic Basis for Disease in Adults and Children. Maryland Heights, MO: Mosby Elsevier; 2010. p.
14. Newhouse RPDearholt SLPoe SSPugh LCWhite KMLast cited on 2015 Jul 18. Available from: http://www.researchgate.net/profile/Robin_Newhouse/publication/8083076_Evidence.based_practice_a_practical_approach_to_implementation/links/00b49519e00eb63b6d000000.pdf .
15. Roth P, Wick W, Weller M. Steroids in neurooncology: Actions, indications, side-effects. Curr Opin Neurol. 2010. 23: 597-602
16. Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965. 18: 298-306
17. Ryken TC, McDermott M, Robinson PD, Ammirati M, Andrews DW, Asher AL. The role of steroids in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010. 96: 103-14
18. Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol. 2015. 17: 488-504
19. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983. 219: 983-5
20. Soffietti R, Cornu P, Delattre JY, Grant R, Graus F, Grisold W. EFNS guidelines on diagnosis and treatment of brain metastases: Report of an EFNS Task Force. Eur J Neurol. 2006. 13: 674-81
21. Spina E, Pisani F, Perucca E. Clinically significant pharmacokinetic drug interactions with carbamazepine.An update. Clin Pharmacokinet. 1996. 31: 198-214
22. Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999. 286: 2511-4
23. Vecht CJ, Hovestadt A, Verbiest HB, van Vliet JJ, van Putten WL. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: A randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994. 44: 675-80
24. Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005. 437: 497-504
25. Woo TM, Wynne AL.editorsPharmacotherapeutics for Nurse Practitioner Prescribers. Philadelphia, PA: F. A. Davis Company; 2012. p.