- Department of Neurosurgery, Dr. Soetomo General Academic Hospital,
- Department of Public Health and Preventive Medicine, Faculty of Medicine Universitas Airlangga,
- Department of Pediatrics, Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
Correspondence Address:
Muhammad Arifin Parenrengi, Department of Neurosurgery, Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
DOI:10.25259/SNI_1204_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dirga Rachmad Aprianto1, Muhammad Arifin Parenrengi1, Budi Utomo2, Asra Al Fauzi1, Eko Agus Subagio1, Ahmad Suryawan3. Comparison of autograft and implant cranioplasty in pediatrics: A meta-analysis. 09-Sep-2022;13:406
How to cite this URL: Dirga Rachmad Aprianto1, Muhammad Arifin Parenrengi1, Budi Utomo2, Asra Al Fauzi1, Eko Agus Subagio1, Ahmad Suryawan3. Comparison of autograft and implant cranioplasty in pediatrics: A meta-analysis. 09-Sep-2022;13:406. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11860
Abstract
Background: Cranioplasty in pediatrics is quite challenging and intricated. The ideal material for it is still debatable until now due to the limited study comparing autologous and implant grafts. This meta-analytic study was conducted to evaluate the risk of infection and revision in pediatric patients after autograft and implant cranioplasty.
Methods: A systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A thorough literature search was conducted on PubMed, Cochrane, Scopus, and ScienceDirect database. Articles published from 2000 to 2021 were selected systematically using PRISMA based on the predetermined eligibility criteria. The relevant data were, then, analyzed and discussed.
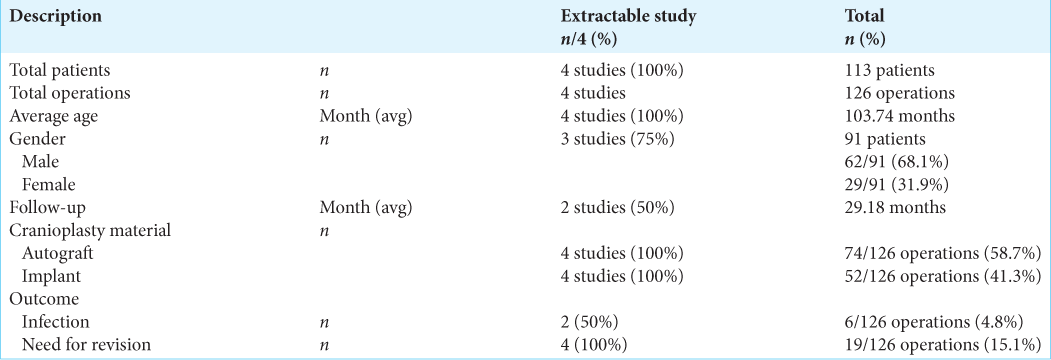
Results: A total of four publications investigating the outcome of autograft and implant cranioplasty were included and reviewed. Postoperative infection and revision rate after 126 cranioplasty procedures (both autograft or implant) from 119 patients below 21 years during time frame of study were analyzed. This meta-analysis study showed that the rate of infection and revision after cranioplasty were not different between the autograft and implant groups.
Conclusion: Autograft and implant cranioplasty have no significant difference in postoperatively infection and revision rate. This study showed that cranioplasty using implant is a plausible option in pediatric patients with cranial defects, depending on the patients’ condition due to similar outcome with autograft cranioplasty. Further studies with larger population and more specific details are necessary to determine the comparison of autograft and implant material in cranioplasty procedure.
Keywords: Autograft cranioplasty, Implant cranioplasty, Infection, Revision
INTRODUCTION
Cranioplasty is widely known as a reconstructive procedure to repair or close a cranial defect. This procedure is generally performed in cases of trauma, tumor, decompressive craniectomy, infection, and congenital abnormalities.[
Autologous bone graft is previously considered as biocompatible, having low risk of infection, as well as allergenic and tend to elicit immune response, thus making autograft preferable than implant in this matter. This procedure, however, has a high rate of bone flap resorption mostly in pediatric patients (below 8 years).[
Several studies have shown that bone replacement materials can be used as alternatives in cranioplasty. Commonly used implant materials for cranioplasty include titanium mesh, polymethylmethacrylate (PMMA), polyetheretherketone (PEEK), and polyethylene.[
The choice of cranioplasty material in pediatric is still debatable. Pediatric patients have characteristics of relatively thinner scalp and calvarial bone than adult, growing cranium size, and limited resources of autologous bone for autograft, thus making cranioplasty more challenging and intricated.[
MATERIALS AND METHODS
Literature search strategy
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.[
Eligibility criteria
We included studies in English or Bahasa language, randomized controlled trial, and prospective or retrospective studies in pediatric patients aged below 21 years who underwent autograft cranioplasty only or implant cranioplasty only. Only studies reporting infection and revision rate in the follow-up period after surgery were included in the study. Reviews, unpublished articles, letters to editor, abstracts, case report or series, and studies not written in English were excluded from the study.
Selection of articles
All records on the title and abstract were screened. Duplicates were then eliminated and three authors (DRA, MAP, and BU) independently assessed potentially relevant studies based on the eligibility criteria. The reasons of exclusion were noted and reported. Included studies for further analysis are summarized in
Assessment of study quality and risk of bias
All included studies for further analysis were independently reviewed by the authors to determine the risk of bias using Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) for nonrandomized studies based on Cochrane Handbook for Systematic Reviews of Interventions. Discrepancies between reviewers were resolved by discussion or involvement of third-party assessors.[
Data extraction
Data extraction from articles was performed independently by two authors (DRA and MAP). The extracted data were collected by the authors, including, year of publication, study design, patient demographic, follow-up period, type of cranioplasty, rate of infection and revision procedure, and graft’s materials. Infection was defined as any occurrence of infection related with the cranioplasty that was reported in selected studies during follow-up period. Revision was defined as any surgical procedure to replace or remove the graft after the cranioplasty procedure.
Statistical analysis
The total number of cranioplasty procedures as well as infection and rate of revision were distinguished based on the types of material (autograft and allograft/implant). Rates of infection and revision were then calculated (pooled) and compared for all materials. A sub-analysis of outcome in autograft method using full-thickness bone flap was performed. Meta-analysis was then performed with RevMan software version 5.4. P < 0.005 was considered statistically significant.[
RESULTS
Study characteristics
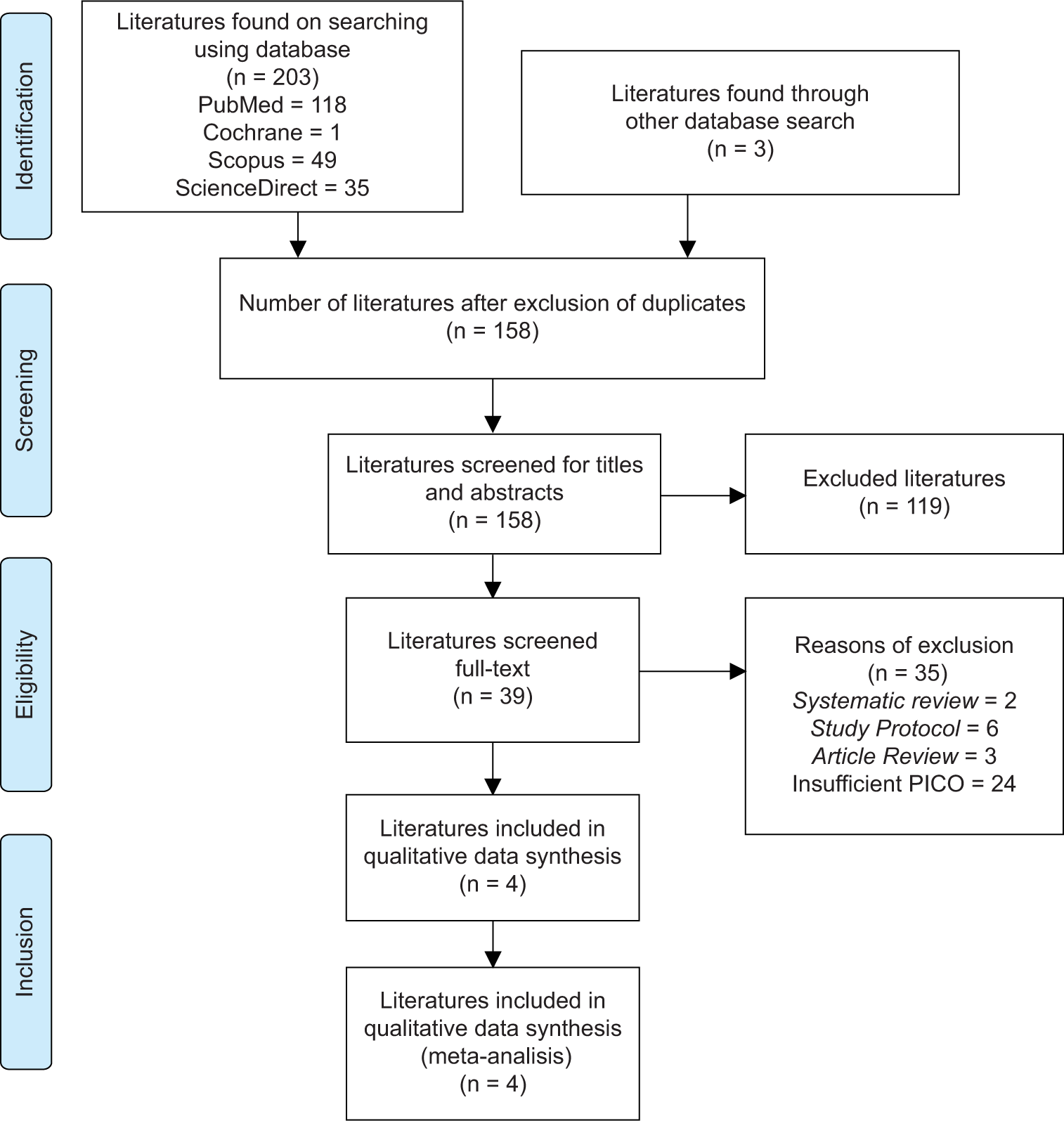
We identified and screened 203 studies including PubMed (118 literature), Cochrane (1 literature), Scopus (49 literature), and ScienceDirect (35 literature). Identification of duplicates and screening through titles and abstracts were carried out resulting in a total of 158 literatures obtained. Full-text screening was then carried out, and 119 literatures were then excluded based on the appropriate eligibility criteria [
Study quality assessment
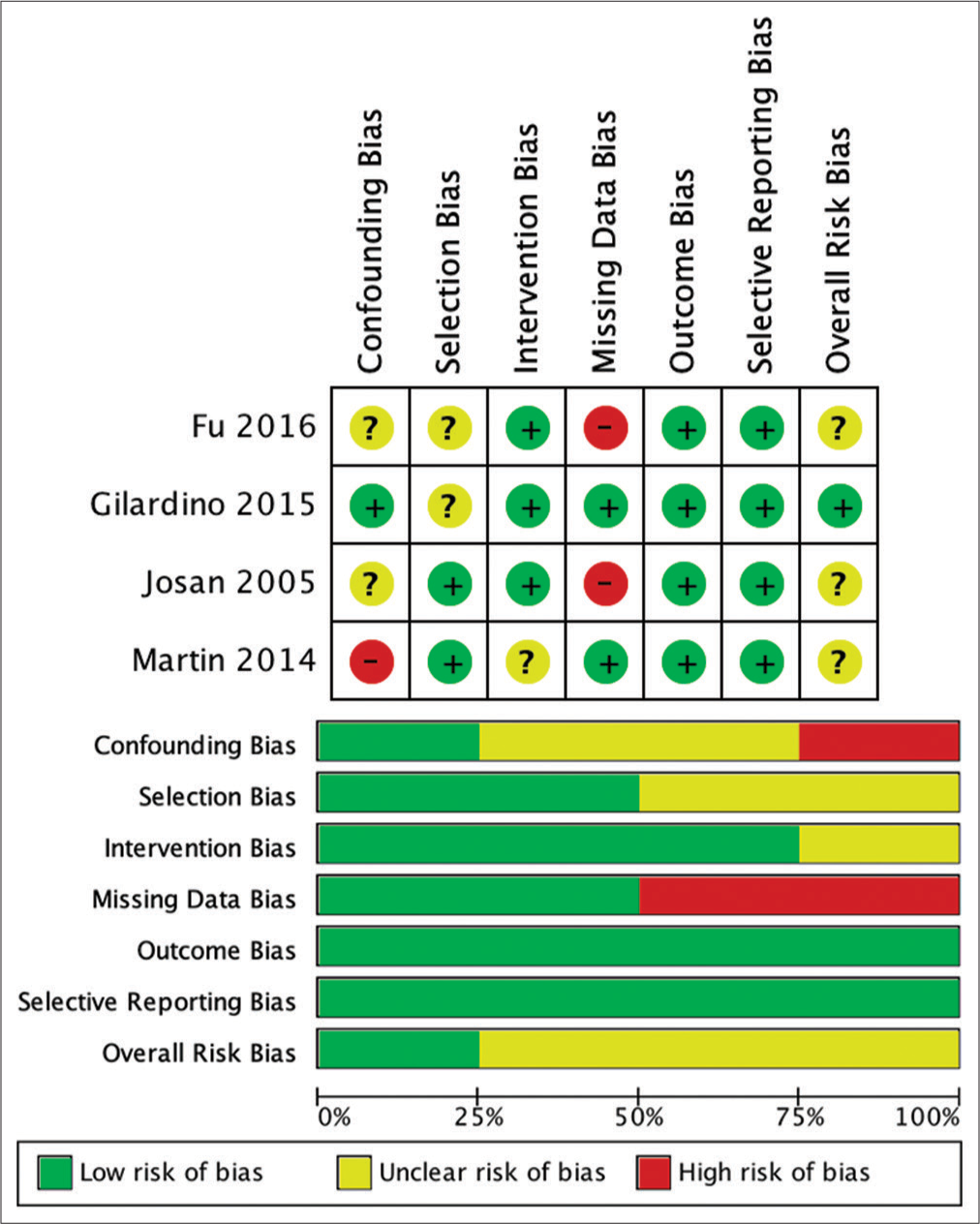
The risk of bias assessment of the involved studies was analyzed using ROBINS-I for nonrandomized. The result of the risk analysis for bias is shown in
Missing data were found to be the highest risk of bias in the studies included in this systematic review. This is due to incomplete patient outcome data that reported in the study by Josan et al. and Fu et al.[
Study results and analysis
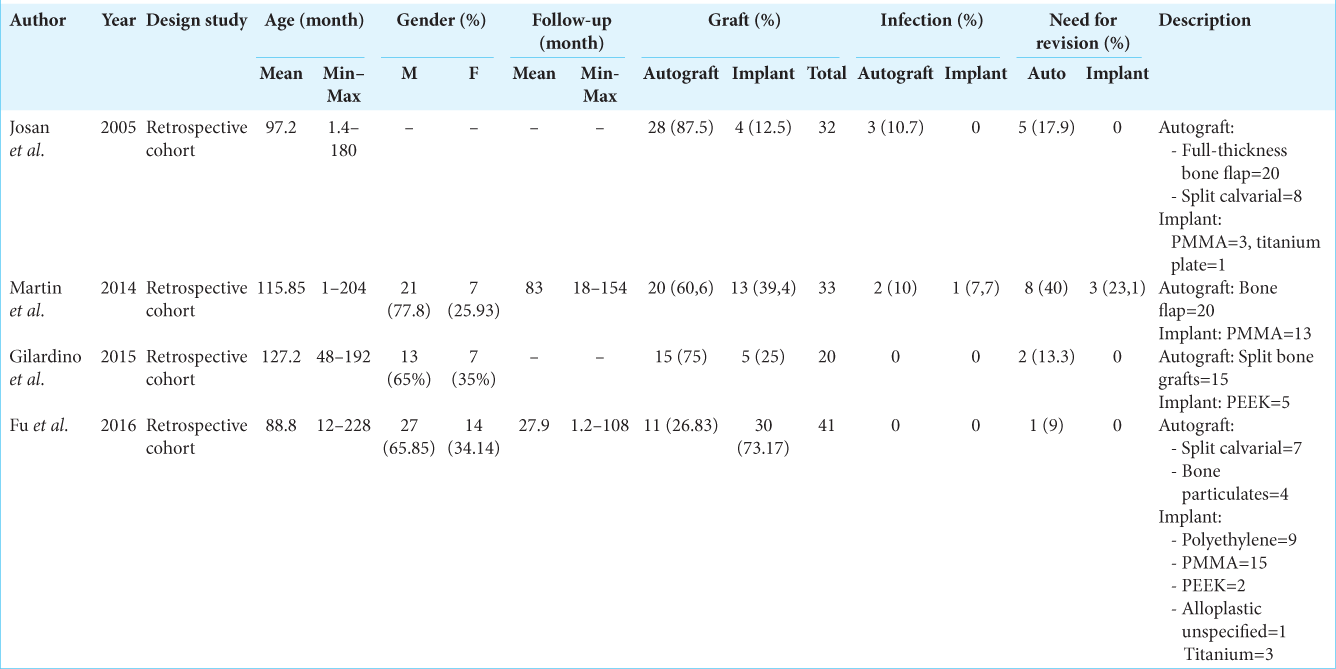
Demographics of all studies included in this meta-analysis are presented in
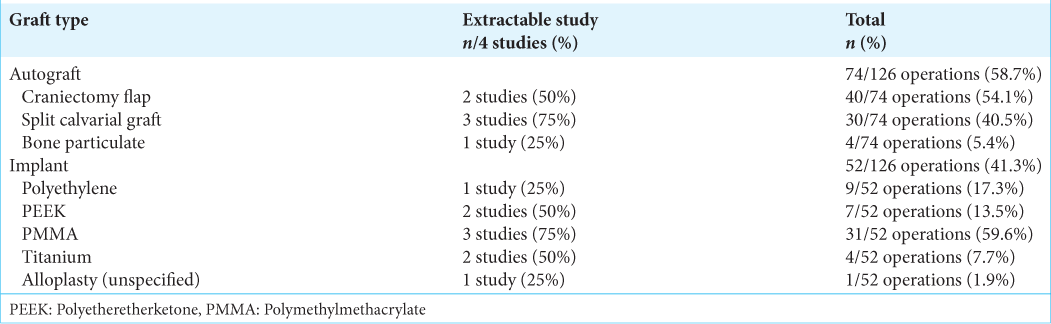
Quantitative analysis showed that patients undergoing autograft cranioplasty had a risk of infection that was not much different from patients undergoing implant cranioplasty (RR = 1.26; 95% CI 0.21–7.46; P = 0.80). The heterogeneity analysis showed that the results were very homogeneous, with an I2 of 0% [
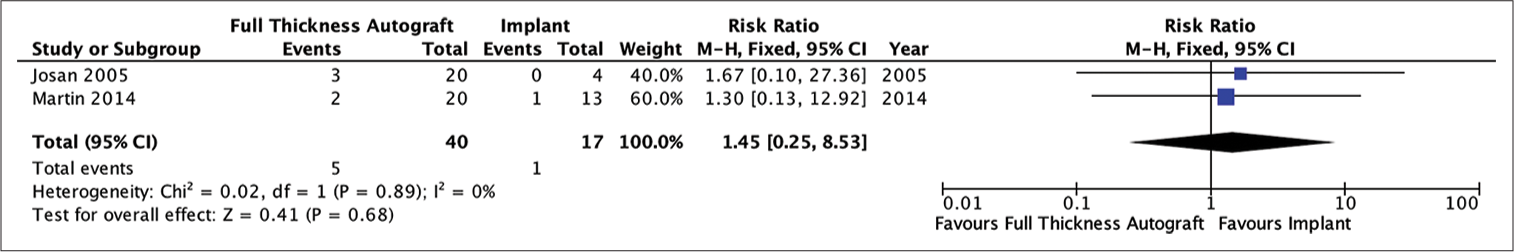
Analysis of the risk ratio for the need for revision of the autograft and implant groups showed that the patients who received autologous bone as a graft for the cranioplasty had no difference risk of requiring revision compared to patients who had implanted cranioplasty (RR = 2.08; 95% CI 0.83–5.25; P = 0.12). The heterogeneity analysis showed that the results were very homogeneous with an I2 of 0% [
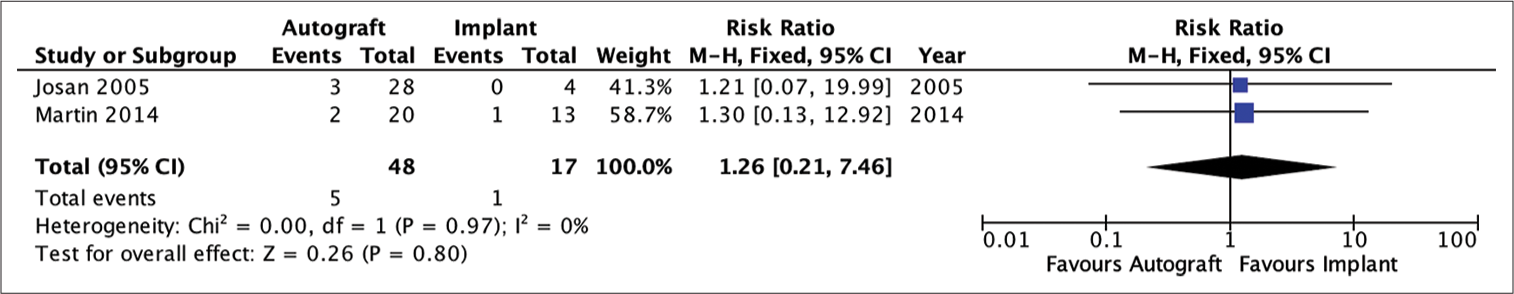
Figure 7:
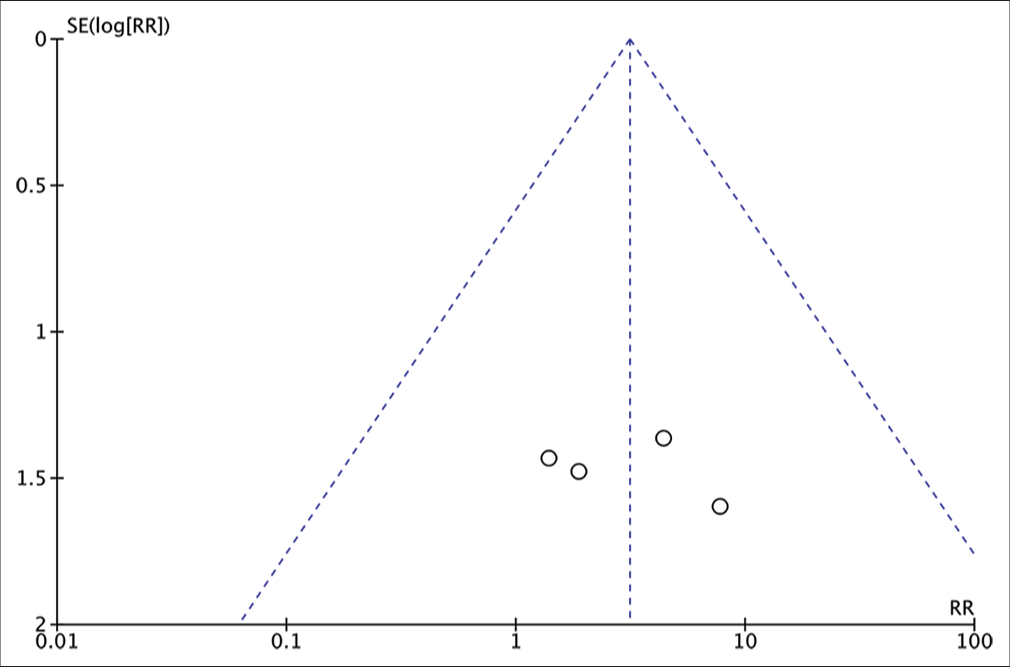
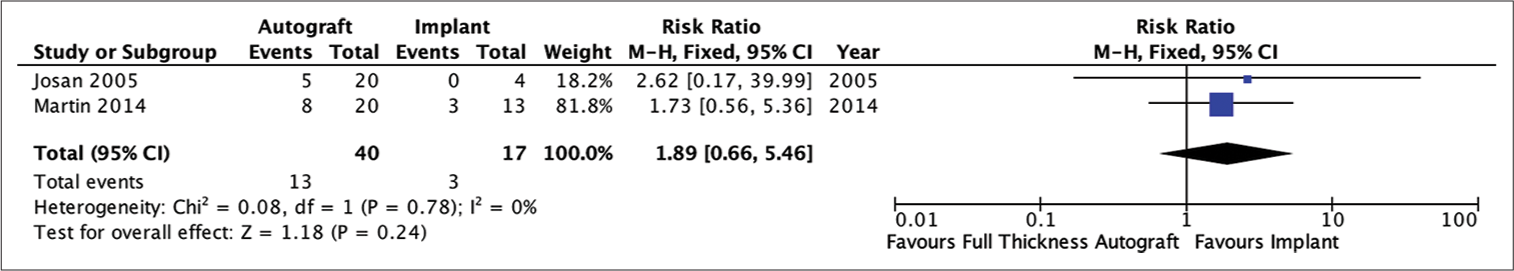
Analysis of the risk ratio requirement for revision of the full-thickness autograft and implant groups. The risk of needing a revision was not much different in the two groups where this result was not statistically significant. The calculation results show a very low heterogeneity (RR = 1.89; 95% CI 0.66e–5.46; P = 0.24; I2 = 0%).
DISCUSSION
Cranioplasty materials can be broadly divided into two types, namely, autologous bone and other bone/implant replacement materials such as PEEK, titanium, polyethylene, and PMMA.[
This review compared the rate of infection between autograft cranioplasty with the implant. The comparison between autograft cranioplasty and implant overall showed that the result was not much different from the infection risk aspect in the two groups where the results of this effect were not statistically significant after being analyzed. Two studies that provide the data related to this are the study by Josan et al. and Martin et al.[
Other materials, namely, implants, also have different characteristics for each material with their respective risks.[
All four studies showed that patients who received autologous bone as a cranioplasty material had no difference in the need of revision than patients who had implanted cranioplasty. Subgroup analysis showed that the risk of needing revision was not significantly different in the full-thickness autograft cranioplasty group with numbers remaining statistically insignificant. Revision cranioplasty is generally performed if there are complications such as infection, osteolysis and resorption of the flap, and lose or broken fixation between the flap and bone so that the flap shifts.
Osteolysis is a major complication of pediatric cranioplasty, 12 of 18 patients (66.7%) developed osteolysis within 6–12 months, and 8 of these patients (44.4%) required follow-up surgery for bone flap replacement with PMMA or CAD cranioplasty. Revised cranioplasty on autograft material can also be caused by nonossification/disintegration of the graft. Osteolysis due to bone flap resorption is a major cause of revision cranioplasty in pediatric patients. Osteolysis is known to resolve spontaneously in some cases, so a “wait and see” procedure may be considered in cases of osteolysis occurring in pediatric patients. The highest rate of bone resorption occurs in the early growth phase to 7 years of age and declines significantly over a period of time between 8 and 14 years. Bowers et al. even reported a younger age of ≤2.5 years age of having high resorption rate. It was hypothesized to be caused by the rapid head growth and high metabolic demand, exceeding bone flap’s capability to fuse with the recipient site,[
We often encounter problems in large cranioplasty defects at our institution using autologous bone graft. High resorption rate limits the area that could be reconstructed if the surgeon chooses to solely use preserved bone graft. Additional bone grafts were often needed from other donor site. This procedure could lead to other potential problem. According to Grant et al., bone flaps larger than 75 cm2 showed up to 60% higher flap resorption rates than smaller (0%) bone flaps.[
Josan et al., in their study, found that 4 out of 24 cases (16.6%) who underwent autograft cranioplasty experienced complications of infection and wound dehiscence that required revision cranioplasty.[
Limitations
There were only limited publications comparing cranioplasty material in pediatric population. Among eligible studies, only one publication provides complete individual data which could make analytic bias due to unrecognized confounding factors. The amount of data may hinder more accurate information interpretation, although we believe that the methodology provided is properly applied in this analysis.
Different time to surgery among papers could also lead to different occurrences of cases (resorption or infection) from different types of intervention. The lack of standardized nor homogeneity among the studies, and possibly in among the subjects of each studies, may be in risk of conclusion misinterpretation, that is, more subjects who experienced infection underwent shorter duration from first surgery to cranioplasty, while those whom require revision may have risk in only one type of bone such as the resorption risk in autologous bone which is not present in implants.
The causes for revision in each subjects from different studies are also not differentiated and analyzed, meaning that different types of grafts with different tendency of complication may affect the analysis, leading to a nonapplicable conclusion.
Unstandardized autologous bone graft storage method between the selected studies could also be a factor for high infection rate. Moreover, the risk of revision may be different in those who received implants due to the growing skull of pediatric patients, and depending on the period of follow-up of each study, this aspect may confoundly affect the outcome of the analysis.[
CONCLUSION
The results of a meta-analysis of four literatures discussing the comparison of the outcomes of autograft and implant cranioplasty in the pediatric population show that outcome of infection did not differ in the implant group compared to the autograft group. The rate of need for revision was also not different in the implant group compared to the autograft group. The results of the subgroup analysis comparing the full-thickness autograft cranioplasty group with the implant cranioplasty group also did not differ between the infection outcome and the need for revision.
The author suggests that implant materials can be used as an option in pediatric patients with cranial defects by taking each patient’s condition into account. Larger, specific studies are needed to determine the comparison of outcomes between autograft cranioplasty and implant cranioplasty in the pediatric population. Other factors that can affect long-term outcomes such as the choice of cranioplasty material from the start, storage and preparation of the material, and the condition of the patient at the start of the cranioplasty procedure also need to be included in further analysis.
Authors’ Contributions
DRA: Conceived and planned the study protocol, search strategy, literature search, systematic review, literature appraisal, data extraction, data synthesis, lead in manuscript writing, and final version of manuscript. MAP: Conceived and planned the study protocol, literature search; systematic review, literature appraisal, data extraction, manuscript writing and editing, and final version of manuscript. BU: Planned the study, conceptualization, search strategy, data extraction, manuscript writing and editing, critical review, and final version of manuscript. AAF: Conceived and planned the study, literature search; systematic review, literature appraisal, data extraction, manuscript writing and editing, final version of manuscript, and submitting for publication. EAS: Conceived and planned the study, literature search; systematic review, literature appraisal, data extraction, manuscript writing and editing, final version of manuscript, and submitting for publication. AS: Conceived and planned the study, literature search; systematic review, literature appraisal, data extraction, manuscript writing and editing, final version of manuscript, and submitting for publication.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank everyone who have contributed to the conceptualization, production, and publication of this study.
References
1. Abu-Ghname A, Banuelos J, Oliver JD, Vyas K, Daniels D, Sharaf B. Outcomes and complications of pediatric cranioplasty: A systematic review. Plast Reconstr Surg. 2019. 144: 433.e-443e
2. Alonso-Rodriguez E, Cebrián JL, Nieto MJ, Del Castillo JL, Hernández-Godoy J, Burgueño M. Polyetheretherketone custom-made implants for craniofacial defects: Report of 14 cases and review of the literature. J Craniomaxillofac Surg. 2015. 43: 1232-8
3. Badhey A, Kadakia S, Mourad M, Inman J, Ducic Y. Calvarial reconstruction. Semin Plast Surg. 2017. 31: 222-6
4. Becker LC, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC. Final report of the cosmetic ingredient review expert panel safety assessment of polymethyl methacrylate (PMMA), methyl methacrylate crosspolymer, and methyl methacrylate/glycol dimethacrylate crosspolymer. Int J Toxicol. 2011. 30: 54S-65
5. Bowers CA, Riva-Cambrin J, Hertzler DA, Walker ML. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr. 2013. 11: 526-32
6. Feroze AH, Walmsley GG, Choudhri O, Lorenz HP, Grant GA, Edwards MS. Evolution of cranioplasty techniques in neurosurgery: Historical review, pediatric considerations, and current trends. J Neurosurg. 2015. 123: 1098-107
7. Frassanito P, Tamburrini G, Massimi L, Di Rocco C, Nataloni A, Fabbri G. Post-marketing surveillance of CustomBone Service implanted in children under 7 years old. Acta Neurochir (Wien). 2015. 157: 115-21
8. Fu KJ, Barr RM, Kerr ML, Shah MN, Fletcher SA, Sandberg DI. An outcomes comparison between autologous and alloplastic cranioplasty in the pediatric population. J Craniofac Surg. 2016. 27: 593-7
9. Goiato MC, Anchieta RB, Pita MS, dos Santos DM. Reconstruction of skull defects: Currently available materials. J Craniofac Surg. 2009. 20: 1512-8
10. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004. 100: 163-8
11. Greenwald JA, Mehrara BJ, Spector JA, Chin GS, Steinbrech DS, Saadeh PB. Biomolecular mechanisms of calvarial bone induction: Immature versus mature dura mater. Plast Reconstr Surg. 2000. 105: 1382-92
12. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ.editors. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, New Jersey: John Wiley and Sons; 2019. p.
13. Inamasu J, Kuramae T, Nakatsukasa M. Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation. J Trauma. 2010. 68: 183-7
14. Josan VA, Sgouros S, Walsh AR, Dover MS, Nishikawa H, Hockley AD. Cranioplasty in children. Childs Nerv Syst. 2005. 21: 200-4
15. Klieverik VM, Miller KJ, Singhal A, Han KS, Woerdeman PA. Cranioplasty after craniectomy in pediatric patients-a systematic review. Childs Nerv Syst. 2019. 35: 1481-90
16. Klinger DR, Madden C, Beshay J, White J, Gambrell K, Rickert K. Autologous and acrylic cranioplasty: A review of 10 years and 258 cases. World Neurosurg. 2014. 82: e525-30
17. Kriegel R, Schaller C, Clusmann H. Cranioplasty for large skull defects with PMMA (Polymethylmethacrylate) or Tutoplast® processed autogenic bone grafts. Zentralbl Neurochir. 2007. 68: 182-9
18. Kwarcinski J, Boughton P, Ruys A, Doolan A, van Gelder J. Cranioplasty and craniofacial reconstruction: A review of implant material, manufacturing method and infection risk. Appl Sci. 2017. 7: 1-17
19. Lee CH, Chung YS, Lee SH, Yang HJ, Son YJ. Analysis of the factors influencing bone graft infection after cranioplasty. J Trauma Acute Care Surg. 2012. 73: 255-60
20. Liu L, Lu ST, Liu AH, Hou WB, Cao WR, Zhou C. Comparison of complications in cranioplasty with various materials: A systematic review and meta-analysis. Br J Neurosurg. 2020. 34: 388-96
21. Martin KD, Franz B, Kirsch M, Polanski W, von der Hagen M, Schackert G. Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir (Wien). 2014. 156: 813-24
22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010. 8: 336-41
23. Piedra MP, Thompson EM, Selden NR, Ragel BT, Guillaume DJ. Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr. 2012. 10: 268-72
24. Rocque BG, Agee BS, Thompson EM, Piedra M, Baird LC, Selden NR. Complications following pediatric cranioplasty after decompressive craniectomy: A multicenter retrospective study. J Neurosurg Pediatr. 2018. 22: 225-32
25. Salam AA, Ibbett I, Thani N. Paediatric cranioplasty: A review. Interdiscip Neurosurg. 2018. 13: 59-65
26. Shah AM, Jung H, Skirboll S. Materials used in cranioplasty: A history and analysis. Neurosurg Focus. 2014. 36: E19
27. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. 355: i4919
28. Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: A systematic review. Neurosurgery. 2011. 68: 1124-9