- Department of Anaesthesiology and Critical Care, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
- Department of Neurosurgery, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
- Department of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
- Department of Neuroanaesthesiology and Neurocritical Care, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India
- Department of Anaesthesiology and Critical Care, MOSC Medical College and Hospital, Kochi, Kerala, India
- Department of Anaesthesiology and Critical Care, All India Institute of Medical Sciences, Bathinda, Punjab, India
Correspondence Address:
Prasanna Udupi Bidkar, Department of Anaesthesiology and Critical Care, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
DOI:10.25259/SNI_892_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Vivek Chandar Chinnarasan1, Prasanna Udupi Bidkar1, Srinivasan Swaminathan1, Manoranjitha Mani2, Balasubramaniyan Vairappan3, Protiti Chatterjee4, Jerry Jame Joy5, Ankita Dey6, Rajasekar Ramadurai1, Adethen Gunasekaran1. Comparison of dexmedetomidine versus fentanyl-based total intravenous anesthesia technique on the requirement of propofol, brain relaxation, intracranial pressure, neuronal injury, and hemodynamic parameters in patients with acute traumatic subdural hematoma undergoing emergency craniotomy: A randomized controlled trial. 13-Dec-2024;15:462
How to cite this URL: Vivek Chandar Chinnarasan1, Prasanna Udupi Bidkar1, Srinivasan Swaminathan1, Manoranjitha Mani2, Balasubramaniyan Vairappan3, Protiti Chatterjee4, Jerry Jame Joy5, Ankita Dey6, Rajasekar Ramadurai1, Adethen Gunasekaran1. Comparison of dexmedetomidine versus fentanyl-based total intravenous anesthesia technique on the requirement of propofol, brain relaxation, intracranial pressure, neuronal injury, and hemodynamic parameters in patients with acute traumatic subdural hematoma undergoing emergency craniotomy: A randomized controlled trial. 13-Dec-2024;15:462. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13287
Abstract
Background: Propofol is one of the most used intravenous anesthetic agents in traumatic brain injury (TBI) patients undergoing emergency neurosurgical procedures. Despite being efficacious, its administration is associated with dose-related adverse effects. The use of adjuvants along with propofol aids in limiting its consumption, thereby mitigating the side effects related to propofol usage. This study aims to compare the safety and efficacy of dexmedetomidine-propofol versus fentanyl-propofol-based total intravenous anesthesia (TIVA) in adult TBI patients.
Methods: A hundred patients posted for emergency evacuation of acute subdural hematoma were enrolled, and they were randomized into two groups of 50 each. Propofol-based TIVA with a Schneider target-controlled infusion model was used for induction and maintenance. Patients in Group F received fentanyl, and those in Group D received dexmedetomidine infusions as adjuvants. Advanced hemodynamic parameters were monitored. Intracranial pressure (ICP) and brain relaxation were measured after dural opening. The mean propofol consumption, number of additional fentanyl boluses, and blood samples for S100b (a biomarker of neuronal injury) were also collected.
Results: The mean propofol consumption in Group D (88.7 ± 31.8 μg/kg/min) was lower when compared to Group F (107.9 ± 34.6 μg/kg/min), (P = 0.005). The mean intraoperative fentanyl requirement and postoperative S100b were significantly reduced in Group D. Subdural ICPs and brain relaxation scores were comparable. Hemodynamic parameters were well maintained in both groups.
Conclusion: In TBI, dexmedetomidine as an adjunct to propofol-based TIVA results in a greater reduction in total propofol consumption and intraoperative opioid requirements while maintaining hemodynamic stability when compared to fentanyl.
Keywords: Brain relaxation, Dexmedetomidine, Propofol, fentanyl, Total intravenous anesthesia, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is a significant cause of morbidity and mortality worldwide.[
Both inhalational and intravenous anesthetic regimens have been used in patients with TBI, with no proven supremacy of one modality over the other. Although inhalational anesthetic agents have been shown to offer neuroprotection, they are often associated with an increase in ICP. Compared to inhalational agents, intravenous anesthesia provides better brain relaxation and ICP control, reduces cerebral metabolic demand, maintains flow-metabolism coupling, and minimizes the risk of cerebral ischemia.[
Propofol is the most common agent used in intravenous anesthesia regimens in TBI. Its beneficial effects in TBI include a reduction in the cerebral metabolic rate of oxygen, CBF reduction, conservation of cerebral autoregulation and carbon dioxide (CO2) responsiveness, maintenance of flow– metabolism coupling, decrease in ICP, and neuroprotection. Despite its efficacy, dose-related adverse effects may occur with propofol, including hemodynamic instability, delayed awakening, platelet dysfunction, and lipemia.[
Fentanyl is a commonly used adjuvant in propofol-based total intravenous anesthesia (TIVA) regimens due to its analgesic efficacy, ability to reduce sympathetic response to airway manipulation and surgical stimulation, and cough suppression.[
Dexmedetomidine is a highly selective α-2 agonist which causes cerebral vasoconstriction, decreases the CBF and cerebral metabolic rate (CMR), thereby conserving flow-metabolism coupling, and helps in reducing ICP.[
TBI is associated with varying degrees of neuronal damage and degeneration, the degree of which determines the severity of TBI.[
Literature regarding the anesthetic management of head injury using TIVA is sparse, with a lack of consensus on the optimal TIVA regimen for TBI patients. This study aims to compare the safety and efficacy of dexmedetomidinepropofol versus fentanyl-propofol-based TIVA in adult TBI patients undergoing craniotomy and evacuation of hematoma in terms of total propofol consumption, ICP, hemodynamic parameters, and biomarkers of neuronal injury.
MATERIALS AND METHODS
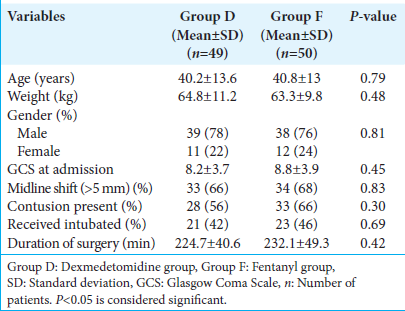
After obtaining approval from the Institute Ethics Committee (JIP/IEC/2021/028), the trial was registered with the Clinical Trials Registry India (CTRI/2021/08/035323). Written informed consent was obtained from a representative family member of subjects satisfying the inclusion and exclusion criteria. One hundred patients belonging to the age group of 18–60 years of either gender, with isolated head injuries, with Glasgow Coma Scale (GCS) <13, scheduled for emergency craniotomy and evacuation of acute traumatic subdural hematoma (SDH) at our hospital were included in the study. Patients with isolated extradural hematoma, hemodynamically unstable patients (defined as heart rate [HR] <50/min and/or systolic blood pressure (SBP) <90 mm Hg before induction of anesthesia), those who were not consenting to partake in the study, and those planned for conservative management were excluded from the study. Preoperative GCS and computed tomography (CT) brain findings were recorded for all the patients.
Sample size
The number of patients to be included was estimated from the average propofol dosage required for maintenance of anesthesia (4.7 ± 1.6 mg/kg/h) when using propofol target-controlled infusion (TCI).[
Randomization
Subjects were randomized into two groups, namely, Group F (fentanyl) and Group D (dexmedetomidine), using a computer-generated randomization table. Allocation concealment was achieved using the serially numbered opaque sealed envelope method. A single researcher who was not involved in data collection or patient follow-up opened the envelope. The study drugs (dexmedetomidine or fentanyl) were administered to the patients according to the group allocation. The anesthesiologist conducting the study and the patients were blinded to the study drugs. Dexmedetomidine and fentanyl were diluted with 0.9% normal saline to a concentration of 2 μg/mL in 50 mL.
In the operation theater, standard monitors, including non-invasive blood pressure, electrocardiogram, and pulse oximetry, were attached, and baseline values were noted. A 16 G/18 G intravenous cannula was secured in one of the accessible veins. A preoperative blood sample for the measurement of S100b was collected and stored.
Group F received fentanyl, and Group D received dexmedetomidine as follows: Intravenous loading dose of 1 μg/kg over 10 min (either fentanyl or dexmedetomidine) followed by an intraoperative maintenance infusion of 0.5 μg/kg/h. After the loading dose of the study drug, anesthesia was induced with fentanyl 1 μg/kg and propofol using a TCI pump with a target effect site concentration of 4–5 μg/mL, in accordance with the Schneider model in both groups (21). Intubation was facilitated with rocuronium 1 mg/kg. Post-induction, patients were intubated with an appropriately sized endotracheal tube. The patients were ventilated with 40% fraction of inspired oxygen (FiO2), and ventilation was adjusted to maintain an end-tidal CO2 (EtCO2) of 30–35 mm Hg.
One of the radial arteries was cannulated with a 20 G cannula. A 7 Fr central line was inserted either in the subclavian vein or the internal jugular vein in all the patients. The cardiac output (CO) monitor (EV1000) was connected to the arterial line and central line, and the values of stroke volume (SV), SV variation (SVV), systemic vascular resistance (SVR), central venous pressure, and CO were obtained. Hemodynamic parameters such as HR, SBP, diastolic blood pressure (DBP), and mean arterial pressure (MAP) were recorded at induction and every minute after induction for 5 min and after that, once in 30 min.
Anesthesia was maintained using propofol with a target plasma concentration between 3 and 4 μg/mL in both groups. In both groups, EtCO2 was maintained at 30–34 mm Hg. HR and invasive blood pressure (IBP) were maintained within 20% of the baseline values.
If the HR and SBP were 20% above the baseline value, a bolus of fentanyl 0.5 μg/kg was given as a rescue measure, followed by an increment in the propofol effect-site concentration by 0.5 μg/mL. The number of additional boluses of fentanyl given was noted. On the contrary, if the HR and SBP were 20% lower, a 200 mL fluid bolus was administered, followed by a reduction in the effect-site concentration of propofol by 0.5 μg/mL. Vasopressors (either phenylephrine or noradrenaline) were given intravenously if the hypotension did not respond to fluids and titration of anesthetics. Normal saline, at the rate of 2 mL/kg/h, was administered in all patients as maintenance fluid.
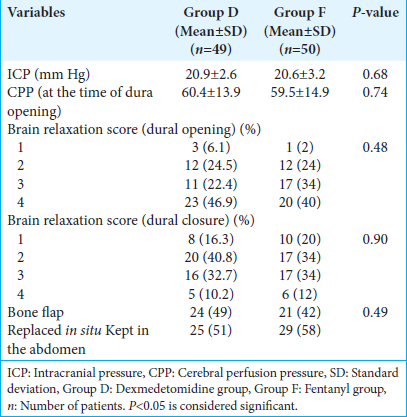
At the time of scalp incision, mannitol 1 g/kg was administered over 20 min. On the creation of the first burr hole, a 22 G/0.8 mm cannula was placed under the dura and connected to a pressure transducer system through a polyethylene catheter. The zero level of ICP was adjusted with the transducer kept at the level of the mastoid process. The pressure measured was taken to be the ICP at that point in time. CPP was calculated as the difference between MAP and ICP. If the ICP was found to be >25 mm Hg, moderate hyperventilation was done to achieve an EtCO2 of 25–28 mm Hg. Additional boluses of mannitol 0.25–0.5 gm/kg were administered if needed. Once the dura was opened, the brain relaxation score was assessed on a four-point scale ([
Statistical analysis
All statistical analyses were carried out using Statistical Package for the Social Sciences Version 29.0 (IBM Corp., Armonk, NY). A frequency and percentage scale were used to express the distribution of categorical characteristics, and continuous variables were expressed as mean ± standard deviation. The comparison of the main outcome variable was done using an independent student t-test. The categorical variables were compared between the groups using the Chi-square test. All statistical analyses were carried out at a significance level of α error of 5%.
RESULTS
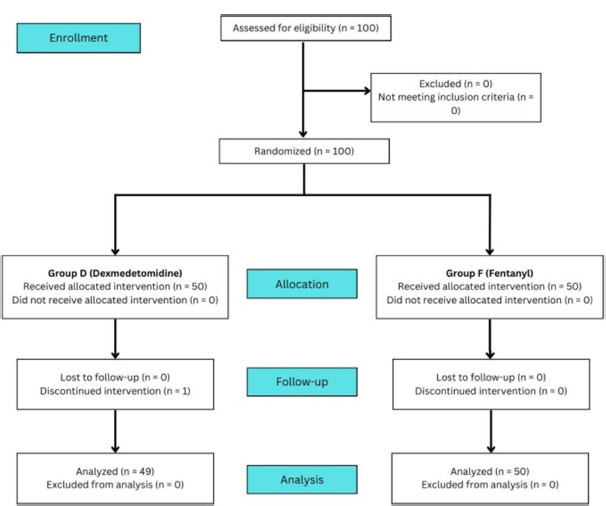
One hundred patients were enrolled in this prospective randomized study. Fifty patients were assigned to each group. One patient in Group D was excluded due to severe persistent bradycardia soon after starting the infusion. Ninety-nine patients (49 in Group D and 50 in Group F) were included in the final analysis [
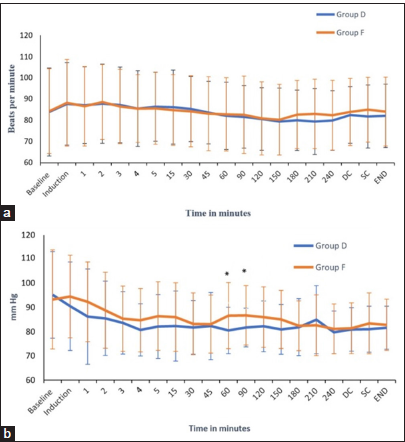
The HR, SBP, DBP, and MAP were recorded at induction, 5 min post-induction, and at 30-min intervals after that. The HR before induction and at various predetermined time points were comparable between the two groups [
DISCUSSION
This randomized controlled trial was formulated to compare dexmedetomidine-based TIVA versus fentanyl-based TIVA in patients with acute traumatic SDH undergoing emergency craniotomy. To the best of our knowledge, there are no studies available comparing the two adjuvants, namely, fentanyl and dexmedetomidine, with propofol in the intraoperative period. The primary outcome of the study was to compare the intraoperative mean propofol consumption between the two groups. Our results demonstrate that the total amount of propofol consumed was lower in the dexmedetomidine-based anesthetic regimen in comparison to the fentanyl-based protocol.
Mean propofol consumption
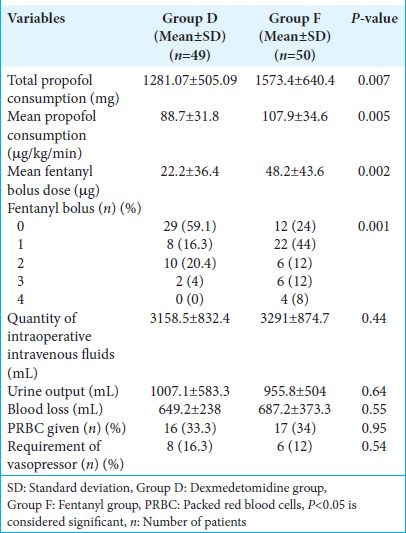
The mean propofol consumption was lower in the dexmedetomidine group (88.7 ± 31.8 μg/kg/min) in comparison to the fentanyl group (107.9 ± 34.6 μg/kg/min) by approximately 18%, and this difference was statistically significant (P < 0.005). In a study by Joy et al., a 30% reduction in the mean propofol consumption was observed with the dexmedetomidine-based anesthetic regimen in patients undergoing elective neurosurgical procedures.[
Chakrabarti et al. studied the effect of dexmedetomidine as an adjunct to propofol on the recovery characteristics and analgesic requirements in patients undergoing cerebellopontine angle surgery under bispectral index (BIS) guidance.[
Brain relaxation and other intraoperative parameters
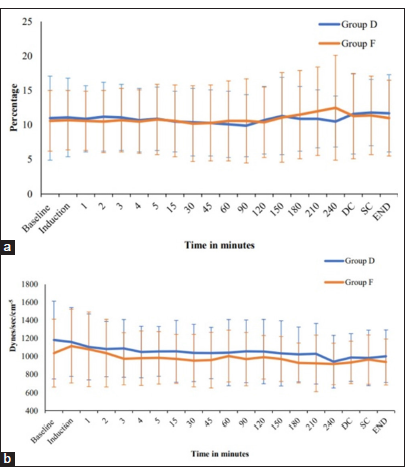
We did not find any difference in brain relaxation and ICP after dural opening between the two groups. Günes et al. compared the hemodynamic profiles, cerebral mechanics, and recovery characteristics of patients undergoing elective neurosurgical procedures receiving dexmedetomidine-remifentanil versus propofol-remifentanil anesthesia.[
Intraoperative blood loss, the total amount of crystalloids, colloids, and blood products administered, and the requirement of vasopressors and blood transfusion were comparable in both groups.
Hemodynamic parameters
Hemodynamic parameters were well maintained in both groups. This finding is reflected in the studies conducted by Song et al. and Tanskanen et al., who did not notice any episodes of bradycardia and/or hypotension while using dexmedetomidine in patients undergoing craniotomy.[
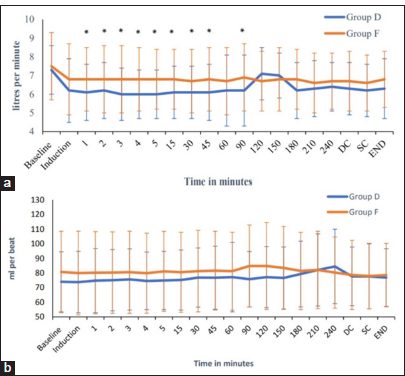
To obtain a more accurate assessment of the hemodynamic status, our study incorporated monitoring of advanced hemodynamic parameters, such as CO, SV, SVV, and SVR [
Additional fentanyl boluses
There was a significant reduction in the additional intraoperative fentanyl requirement in the dexmedetomidine group in comparison to the fentanyl group in our study. Joy et al. and Chakrabarti et al. demonstrated similar results in their study, where the total opioid consumption was significantly lower in the dexmedetomidine group.[
That being said, few other studies have demonstrated no significant reduction in intraoperative opioid usage with the addition of dexmedetomidine.[
Brain biomarkers
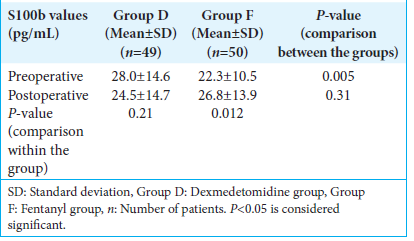
The baseline levels of S100b were elevated in the dexmedetomidine group, as compared to the fentanyl group. However, there was a reduction in the postoperative biomarker values in the dexmedetomidine group, a finding that can be attributed to the neuroprotective and anti-apoptotic effects of the drug.[
Their study reported lower perioperative values of S100b in patients receiving dexmedetomidine. In a meta-analysis of 879 randomized controlled trials, dexmedetomidine was shown to reduce the surges in S100b values and to mitigate the stress-related increases in HR, MAP, and ICP in patients with ischemic brain injury. Literature regarding similar effects of dexmedetomidine in patients with TBI is lacking. To the best of our knowledge, this is the first study reporting a plausible neuroprotective role of intraoperative dexmedetomidine in TBI patients.
Course of hospital stay
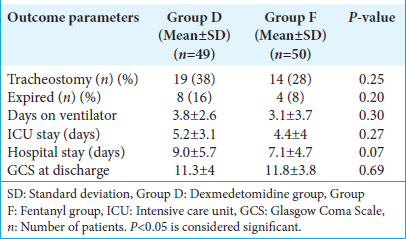
The length of ICU stays, number of ventilator days, length of hospitalization, number of patients requiring tracheostomy, and the in-hospital mortality were comparable between the two groups in our study [
Limitations of the study
Our study is unique in exploring the neuroprotective role and propofol-sparing effects of dexmedetomidine, among other outcome measures, in the setting of TBI. Nevertheless, our study has certain limitations. We did not measure the plasma concentrations of propofol, dexmedetomidine, or fentanyl. Furthermore, the comparison of the cost-effectiveness of the study drugs was not part of our study. Considering the relatively short duration of action of the study drugs and the lack of significant long-term effects on the outcome measures, the follow-up was not extended beyond patient discharge from the hospital.
CONCLUSION
Based on the findings of our study, we conclude that dexmedetomidine, when used as an adjunct to propofol-based TIVA, results in a greater reduction in the total propofol consumption and intraoperative opioid requirement while maintaining hemodynamic stability when compared to fentanyl. Subdural ICPs, brain relaxation scores at dural opening, dural closure, and after evacuation of hematoma were comparable between the two groups. There was no statistically significant difference between the two groups with respect to perioperative fluid replacement, requirement of vasopressors, blood loss, and blood transfusion. Although the MAP and CO were lower in the dexmedetomidine group, they were well within the normal limits. The two groups were comparable in terms of other hemodynamic parameters, such as SV, SVV, and SVR. Serum levels of S100b showed a reduction from their baseline value in the dexmedetomidine group, supporting the neuroprotective role of dexmedetomidine in TBI. Further studies are needed to confirm the neuroprotective role of dexmedetomidine in TBI patients.
Transparency, rigor, and reproducibility summary
The study design and analysis plan were preregistered on August 2, 2021, at the Clinical Trials Registry India (CTRI/2021/08/035323). The prespecified sample size was 50 per group, yielding a statistical power of 80% for the detection of a 20% reduction in the total propofol requirement in patients who received dexmedetomidine and in those who received fentanyl as an adjuvant. All subjects were assigned to Group F (fentanyl) and Group D (dexmedetomidine) using a computer-generated randomization table, yielding groups that did not differ in baseline characteristics. One hundred subjects were enrolled, and primary outcomes were assessed in 99 subjects (50 in Group F, 49 in Group D) after excluding 1 patient in Group D due to severe persistent bradycardia soon after the commencement of drug infusion. All primary outcomes were assessed by investigators blinded to group assessment. The data and analytic code have not been deposited at any external site due to hospital policy but are partially available on request. The findings have not yet been replicated or externally validated. The manuscript is open-access.
Author contributions
VC: Methodology, Investigation, Writing-original draft; PB: Conceptualization, Methodology, Supervision; SS: Conceptualization, Visualization, Supervision; MK: Visualization, Supervision; BV: Data curation, Supervision; PC: Methodology, Writing-review, and editing; JJ: Data curation, Formal analysis; RR: Methodology, Writing-review, and editing.
Ethical approval
The research/study approved by the Institutional Review Board at Jawaharlal Institute of Postgraduate Medical Education and Research, number JIP/IEC/2021/028, dated July 06, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The study was supported by intramural funds from the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER/01/123/2021/01555).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary material on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ali AR, El Ghoneimy MN. Dexmedetomidine versus fentanyl as adjuvant to propofol: Comparative study in children undergoing extracorporeal shock wave lithotripsy. Eur J Anaesthesiol. 2010. 27: 1058-64
2. Andleeb R, Agrawal S, Gupta P. Evaluation of the effect of continuous infusion of dexmedetomidine or a subanesthetic dose ketamine on transcranial electrical motor evoked potentials in adult patients undergoing elective spine surgery under total intravenous anesthesia: A randomized controlled exploratory study. Asian Spine J. 2022. 16: 221-30
3. Beule AG, Wilhelmi F, Kühnel TS, Hansen E, Lackner KJ, Hosemann W. Propofol versus sevoflurane: Bleeding in endoscopic sinus surgery. Otolaryngol Neck Surg. 2007. 136: 45-50
4. Bindra A, Kaushal A, Prabhakar H, Chaturvedi A, Chandra PS, Tripathi M. Neuroprotective role of dexmedetomidine in epilepsy surgery: A preliminary study. Neurol India. 2019. 67: 163-8
5. Chakrabarti D, Kamath S, Madhusudan Reddy KR, Srinivas DB, Manohar N, Masapu D. Effect of adjunctive dexmedetomidine on anesthesia and analgesia requirement and recovery characteristics during Bispectral Index-guided anesthesia for cerebello-pontine angle surgeries: A randomized clinical trial. J Anaesthesiol Clin Pharmacol. 2018. 34: 496-502
6. Cravens GT, Packer DL, Johnson ME. Incidence of propofol infusion syndrome during noninvasive radiofrequency ablation for atrial flutter or fibrillation. Anesthesiology. 2007. 106: 1134-8
7. Demlie TA, Alemu MT, Messelu MA, Wagnew F, Mekonen EG. Incidence and predictors of mortality among traumatic brain injury patients admitted to Amhara region Comprehensive Specialized Hospitals, northwest Ethiopia, 2022. BMC Emerg Med. 2023. 23: 55
8. Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003. 60: 540-51
9. Fragen RJ, Fitzgerald PC. Effect of dexmedetomidine on the minimum alveolar concentration (MAC) of sevoflurane in adults age 55 to 70 years. J Clin Anesth. 1999. 11: 466-70
10. Gopalakrishna KN, Dash PK, Chatterjee N, Easwer HV, Ganesamoorthi A. Dexmedetomidine as an anesthetic adjuvant in patients undergoing transphenoidal resection of pituitary tumor. J Neurosurg Anesthesiol. 2015. 27: 209-15
11. Günes Y, Gündüz M, Özcengiz D, Özbek H, Isik G. Dexmedetomidine-remifentanil or propofol-remifentanil anesthesia in patients undergoing intracranial surgery. Neurosurg Q. 2005. 15: 122
12. Hajduková L, Sobek O, Prchalová D, Bílková Z, Koudelková M, Lukášková J. Biomarkers of brain damage: S100B and NSE concentrations in cerebrospinal fluid-a normative study. Biomed Res Int. 2015. 2015: 379071
13. Heybati K, Zhou F, Ali S, Deng J, Mohananey D, Villablanca P. Outcomes of dexmedetomidine versus propofol sedation in critically ill adults requiring mechanical ventilation: A systematic review and meta-analysis of randomised controlled trials. Br J Anaesth. 2022. 129: 515-26
14. Ingebrigtsen T, Romner B. Biochemical serum markers of traumatic brain injury. J Trauma Acute Care Surg. 2002. 52: 798
15. Jasmitha K, Hemanth N, Samantaray A. A comparative study of dexmedetomidine-ropofol and fentanyl-propofol on perioperative haemodynamics, propofol requirement and post-operative recovery profile in patients undergoing elective abdominal surgeries-A prospective randomised double-blind study. J Clin Sci Res. 2022. 11: 94
16. Joy JJ, Bidkar PU, Swaminathan S, Balasubramanian M, Dey A, Chinnarasan VC. Comparison of dexmedetomidine versus fentanyl-based anesthetic protocols under patient state index guidance in patients undergoing elective neurosurgical procedures with intraoperative neurophysiological monitoring. Cureus. 2023. 15: e35864
17. Kanda H, Kunisawa T, Kurosawa A, Nagashima M, Suzuki A, Takahata O. Effect of dexmedetomidine on anesthetic requirements in cardiovascular surgery. Masui. 2009. 58: 1496-500
18. Kang WS, Kim SY, Son JC, Kim JD, Muhammad HB, Kim SH. The effect of dexmedetomidine on the adjuvant propofol requirement and intraoperative hemodynamics during remifentanil-based anesthesia. Korean J Anesthesiol. 2012. 62: 113-8
19. Kannabiran N, Bidkar PU. Total intravenous anesthesia in neurosurgery. J Neuroanaesth Crit Care. 2018. 5: 141-9
20. Kunisawa T, Ueno M, Kurosawa A, Nagashima M, Hayashi D, Sasakawa T. Dexmedetomidine can stabilize hemodynamics and spare anesthetics before cardiopulmonary bypass. J Anesth. 2011. 25: 818-22
21. Li F, Wang X, Deng Z, Zhang X, Gao P, Liu H. Dexmedetomidine reduces oxidative stress and provides neuroprotection in a model of traumatic brain injury via the PGC-1α signaling pathway. Neuropeptides. 2018. 72: 58-64
22. Liu N, Chazot T, Hamada S, Landais A, Boichut N, Dussaussoy C. Closed-loop coadministration of propofol and remifentanil guided by bispectral index: A randomized multicenter study. Anesth Analg. 2011. 112: 546-57
23. Magni G, Baisi F, La Rosa I, Imperiale C, Fabbrini V, Pennacchiotti ML. No difference in emergence time and early cognitive function between sevoflurane-fentanyl and propofol-remifentanil in patients undergoing craniotomy for supratentorial intracranial surgery. J Neurosurg Anesthesiol. 2005. 17: 134-8
24. Ng SY, Lee AY. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019. 13: 528
25. Ngwenyama NE, Anderson J, Hoernschemeyer DG, Tobias JD. Effects of dexmedetomidine on propofol and remifentanil infusion rates during total intravenous anesthesia for spine surgery in adolescents. Paediatr Anaesth. 2008. 18: 1190-5
26. Pascoe PJ, Ilkiw JE, Frischmeyer KJ. The effect of the duration of propofol administration on recovery from anesthesia in cats. Vet Anaesth Analg. 2006. 33: 2-7
27. Pérez de Arriba N, Antuña Ramos A, Martin Fernandez V, Rodriguez Sanchez MD, Gonzalez Alarcon JR, Alvarez Vega MA. Risk factors associated with inadequate brain relaxation in craniotomy for surgery of supratentorial tumors. Cureus. 2022. 14: e25544
28. Preethi J, Bidkar PU, Cherian A, Dey A, Srinivasan S, Adinarayanan S. Comparison of total intravenous anesthesia vs. inhalational anesthesia on brain relaxation, intracranial pressure, and hemodynamics in patients with acute subdural hematoma undergoing emergency craniotomy: A randomized control trial. Eur J Trauma Emerg Surg. 2021. 47: 831-7
29. Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998. 88: 1170-82
30. Sen S, Chakraborty J, Santra S, Mukherjee P, Das B. The effect of dexmedetomidine infusion on propofol requirement for maintenance of optimum depth of anaesthesia during elective spine surgery. Indian J Anaesth. 2013. 57: 358
31. Soliman RN, Hassan AR, Rashwan AM, Omar AM. Prospective, randomized study to assess the role of dexmedetomidine in patients with supratentorial tumors undergoing craniotomy under general anaesthesia. Middle East J Anaesthesiol. 2011. 21: 325-34
32. Song J, Ji Q, Sun Q, Gao T, Liu K, Li L. The opioid-sparing effect of intraoperative dexmedetomidine infusion after craniotomy. J Neurosurg Anesthesiol. 2016. 28: 14-20
33. Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumor surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006. 97: 658-65
34. Vokes DE, Linskey ME, Armstrong WB. Propofol lipemia mimicking chyle leak during neck dissection. Head Neck. 2006. 28: 1147-9
35. Walia C, Gupta R, Kaur M, Mahajan L, Kaur G, Kaur B. Propofol sparing effect of dexmedetomidine and magnesium sulfate during BIS targeted anesthesia: A prospective, randomized, placebo controlled trial. J Anaesthesiol Clin Pharmacol. 2018. 34: 335
36. Walker CT, Kim HJ, Park P, Lenke LG, Weller MA, Smith JS. Neuroanesthesia guidelines for optimizing transcranial motor evoked potential neuromonitoring during deformity and complex spinal surgery: A Delphi consensus study. Spine. 2020. 45: 911-20
37. Wang X, Ji J, Fen L, Wang A. Effects of dexmedetomidine on cerebral blood flow in critically ill patients with or without traumatic brain injury: A prospective controlled trial. Brain Inj. 2013. 27: 1617-22
38. Wang X. Neuroprotective effects and mechanisms of fentanyl preconditioning against brain ischemia. Crit Care. 2006. 10: P446
39. Wu X, Hang LH, Chen YF, Wang H, Shao DH, Chen Z. Remifentanil requirements for preventing motor response to skin incision in healthy women anesthetized with combinations of propofol and dexmedetomidine titrated to similar Bispectral Index (BIS) values. Ir J Med Sci. 2015. 184: 805-11