- Department of Neurosurgery, Arrowhead Regional Medical Center, 400 N Pepper Avenue, Colton, United States.
- Department of Neurosurgery, Riverside University Health System, 26520 Cactus Avenue, Moreno Valley, California - 92555, United States.
Correspondence Address:
Marc Billings
Department of Neurosurgery, Arrowhead Regional Medical Center, 400 N Pepper Avenue, Colton, United States.
Department of Neurosurgery, Riverside University Health System, 26520 Cactus Avenue, Moreno Valley, California - 92555, United States.
DOI:10.25259/SNI-82-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Marc Billings, Robert Dahlin, Bailey Zampella, Raed Sweiss, Shokry Lawandy, Dan Miulli. Conditions associated with giant pituitary tumors at the time of surgery effecting outcome morbidity and mortality. 07-Jun-2019;10:92
How to cite this URL: Marc Billings, Robert Dahlin, Bailey Zampella, Raed Sweiss, Shokry Lawandy, Dan Miulli. Conditions associated with giant pituitary tumors at the time of surgery effecting outcome morbidity and mortality. 07-Jun-2019;10:92. Available from: http://surgicalneurologyint.com/surgicalint-articles/9355/
Abstract
Background:Surgical outcome prediction has assisted physicians in discussing surgical intervention or expectant management. While increasing pituitary tumor size would seem to be associated with increasing challenge of removal and associated complications, that relationship has not been borne in the literature.
Methods:We performed a retrospective review of a consecutive cohort of pituitary surgeries completed at our institution. Data included age at the time of surgery, presenting symptoms and Glasgow Coma scale (GCS), GCS at discharge or 7 days postoperatively, GCS at 6 months, adenoma size, imaging characteristics of the tumor and brain before resection, postoperative complications, the presence of preoperative hydrocephalus, brainstem compression, and patient mortality.
Results:Patients with giant adenomas were more likely to present with a cranial nerve palsy (P = 0.019), altered mental status (P = 0.0001), hydrocephalus (P = 0.002), and mass effect on the brainstem (P = 0.020). Patients who experienced a postoperative decline in mental status were more likely to present with altered mental (P = 0.006), had an increased prevalence of mass effect on the brainstem (P = 0.005), and were more likely to have either an ischemic stroke (P = 0.0001) and vasospasms or new intraparenchymal hemorrhage (P = 0.013).
Conclusion:The results of this study demonstrate that postoperative mental status declines after pituitary adenoma resection can be directly related to brainstem compression and further surgical irritation of the surrounding vasculature. The intraoperative irritation can be multifactorial and may result as the decompressed brain structures assume their anatomical position.
Keywords: Brainstem compression, Hydrocephalus, Pituitary tumors, Vasospasm
INTRODUCTION
Standard nomenclature used to describe pituitary adenomas is primarily based on size. Adenomas <10 mm are described as microadenomas, adenomas >10 mm are macroadenomas, adenomas >30 mm are large adenomas, and adenomas >40 mm are considered as giant pituitary adenomas. While it would seem logical that increasing tumor size would be associated with the increasing challenge of removal and associated complications, there is a paucity of literature that investigates this relationship. To date, there has been only one study that concluded that increased size was correlated with increased mortality,[
The current literature is unclear as to whether there exists a statistical difference between complications in adenoma resection of all sizes versus giant adenomas. One problem with the current literature is that a large series of pituitary resections do not specify complication rates based on size, and hence, the data are grouped together and unable to be compared to studies specifically of giant adenomas. Another barrier within the literature is that most papers specifically analyzing complications of giant have a rather small sample size limiting statistical significance.
In the current literature for giant adenomas, the rate of diabetes insipidus (DI) ranges from 1.7% to 9.8%[
While most case series discuss their operative mortality, to date, no study has attempted to address functional outcome by both pre- and postoperative Glasgow Coma Scale (GCS), Glasgow outcome scale, or Karnofsky Performance Score (KPS). Leung retrospectively reviewed 12 patients who underwent resection of either large or giant pituitary adenomas, and within their study group, only 25% of patients had a KPS of 100 at final follow-up. However, this study did not record preoperative KPS for comparison.[
This study was undertaken to evaluate the possible correlations between tumor size and their associated surgical challenges and complication rates, as well as to determine whether characteristics of either the patient or tumor, other than tumor size, correlated with a change in functional status.
METHODS
Research approval was performed by the IRB at Arrowhead Regional Medical Center. A search of the neurosurgical database that is maintained at the hospital was performed to identify all patients undergoing resection of a pituitary adenoma consecutively from 2009 to 2015. Data to be recorded included the patients age at the time of surgery, presenting symptoms and GCS, GCS at discharge or 7 days postoperatively, GCS at 6 months, size of the pituitary adenoma, imaging characteristics of the tumor and brain before resection, postoperative complications, and patient mortality. Primary outcomes were mortality and 6 months’ GCS. Patients >18 years old with a pathologically proven diagnosis of pituitary adenoma resected by any surgical approach were included in the study. The only exclusion criterion for the study was insufficient documentation of the above data points.
Imaging characteristics measured included the superior/inferior diameter of the tumor measured by a mid-sagittal magnetic resonance imaging (MRI) from the floor of the sella turcica to the superior end of tumor. Anteroposterior dimensions were obtained by measuring the maximal diameter of the tumor in the plane that is perpendicular to the line used in measuring the superior/inferior diameter. Maximal medial/lateral diameter was calculated as the maximal mediolateral diameter of the tumor on axial view MRI. The presence of preoperative hydrocephalus was evaluated on preoperative MRI as well as the presence of mass effect on the brainstem as evidence by deflection of the normal brainstem structures was also recorded. A single observer made all measurements.
Statistical analysis was performed using SPSS version 20. Chi-square analysis was performed to determine differences between qualitative data, and independent Student’s t-test was chosen to evaluate the difference between quantitative data with P < 0.05 being considered statistically significant.
RESULTS
Two clinical determinations are important when reviewing the data. Does there exists a size after which complications become more frequent; and after identifying those patients who did not do well, are we able to identify risk factors that portend a worse prognosis?
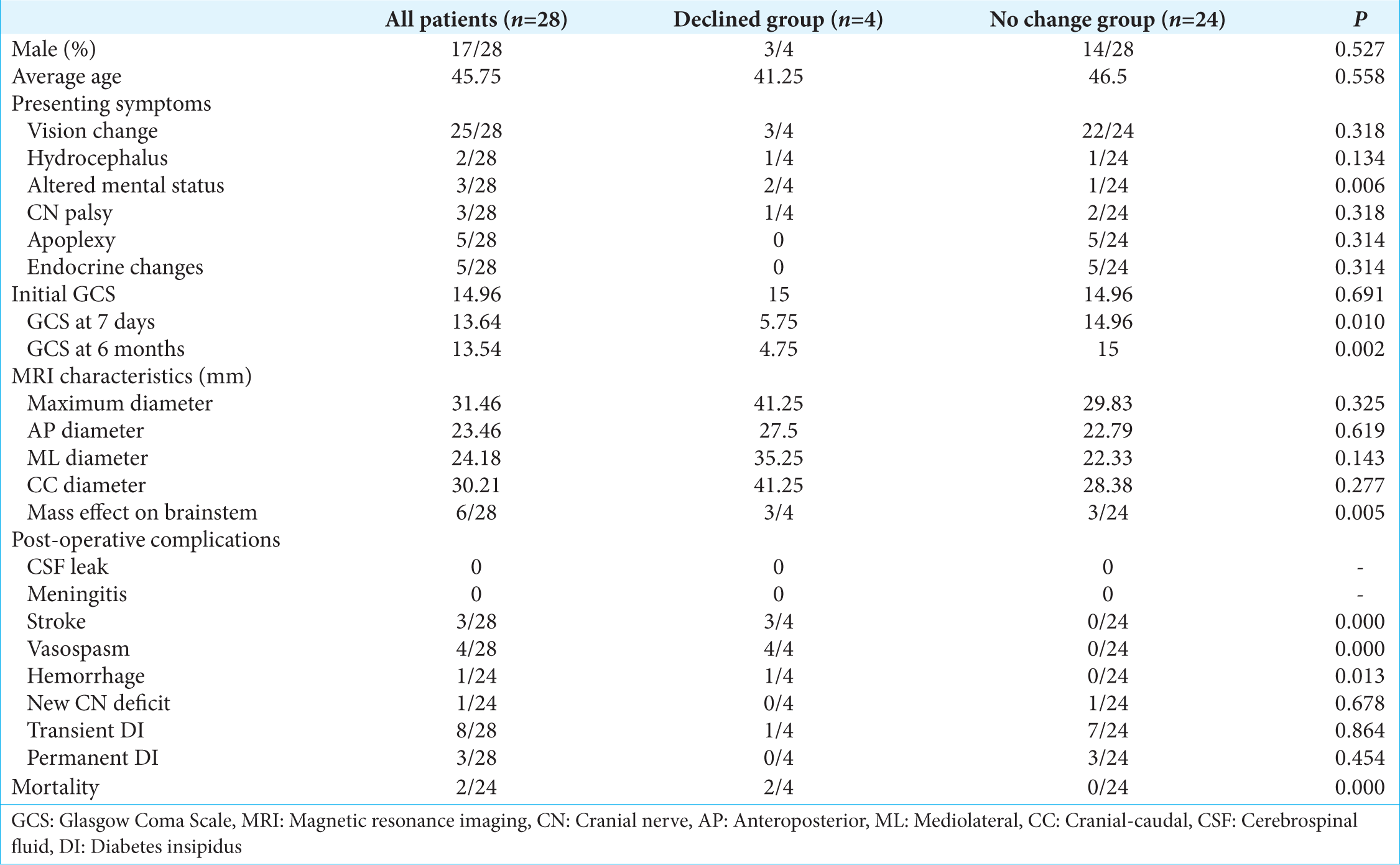
In reviewing the data with regard to size, with macroadenomas defined as adenomas >1 cm but <4 cm, and giant adenomas being defined as adenomas >4 cm in maximal diameter, there were a total of five giant adenomas in our series of patients. There was no difference in age or gender between patients with macro or giant adenomas. Patients with giant adenomas were, however, more likely to present with a cranial nerve (CN) palsy (P = 0.019), altered mental status (P = 0.0001), hydrocephalus (P = 0.002), or mass effect on the brainstem (P = 0.020). There were no differences in the rates of visual changes, apoplexy, or endocrine dysfunction on presentation. Postoperatively, macroadenomas had a postoperative day 7 GCS of 14.22 versus 11.0 (P = 0.253), and a 6 month postoperative GCS of 14.13 versus 10.80 (P = 0.276). There was an increased rate of stroke in patients with giant adenomas (P = 0.019). There was no difference in the rate of postoperative transient DI, permanent DI, CN palsies, hemorrhage, or mortality.
When comparing patients who had a persistently depressed GCS at 6 months versus patients who either improved neurologically or remained at their baseline, there was no difference in age or sex between the two groups. Furthermore, there was no difference between the two groups in the rate of apoplexy, vision changes, endocrine changes, CN palsies, or hydrocephalus on presentation.
The average age of patients who experienced a decline in mental status versus no change was 41.25 versus 46.5 (P = 0.558). The mean maximum diameter of the adenoma in patients who experienced a decline in mental status was 41.25 versus 29.83 (P = 0.325). Other dimensions such as maximum cranial caudal diameter, mediolateral diameter, and anteroposterior diameter were not significantly different.
Patients with mass effect on the brainstem were more likely to experience a decline in mental status (P = 0.005) and present with an episode of altered mental status (P = 0.006). Postoperatively, all patients who had a decline in mental status were found to have either an ischemic stroke (P = 0.0001) or new intraparenchymal hemorrhage (P = 0.013). The postoperative decline in GCS, as evidenced by ischemic or hemorrhagic stroke, was only seen in patients with giant adenomas that had brain stem compression and an episode of altered mental status preoperatively. A preoperative altered mental status was not seen in all giant adenomas; however, all patients with a preoperative altered mental status had a giant adenoma and also a preoperative altered mental status was not seen in all patients with brainstem compression. All patients with postoperative mental status decline had documented vasospasms on transcranial Doppler. However, testing for vasospasms was only conducted in patients with postoperative mental status decline. This data is summarize in
DISCUSSION
Tumors resected through a craniotomy typically have characteristics that make them poor candidates for resection through other methods due to the inability to achieve maximal resection due to either its size or pattern of spread which makes parts of the tumor inaccessible through a transnasal approach. This pattern was reflected in our study in which only two craniotomies had been conducted, with both being performed on patients with giant adenomas. There was a trend toward patients experiencing a decline in GCS postoperatively as the size of the adenoma increased; however, this was not statistically significant.
Patients who experienced a decline in mental status postoperatively presented with a preoperative episode of altered mental status had brainstem compression from a pituitary adenoma, cerebral vasospasms, and either a postoperative ischemic stroke or intraparenchymal hemorrhage.
Patients who presented with an episode of altered mental status (P = 0.006) and mass effect on the brainstem were more likely to experience a postoperative decline in mental status (P = 0.005) and/or death (P = 0.005). The patients who had a mass effect on the brainstem and experienced a decline due to infarction also had cerebral vasospasm on the side of the infarction. A tumor that is either invested in, adherent to, or significantly displacing the circle of Willis causing injury or spasm of these arteries preoperatively can be exacerbated during surgical resection.
CONCLUSION
The results of this retrospective cohort study demonstrate that postoperative mental status declines after giant pituitary adenoma resection can be directly related to brainstem compression and further surgical irritation of the surrounding vasculature. The intraoperative irritation can be multifactorial and may result as the decompressed brain structures assume their anatomical position.
While we found that only patients with preoperative altered mental status, giant pituitary adenoma, and mass effect on the brainstem were significantly more likely to experience a decline in mental status postoperatively, this was identified within a small sample size . Further, as we did not have many patients who underwent craniotomy as a means of resection, and thus we were unable to control for this confounding variable. At this time, future studies will need to be performed to determine whether these variables do indeed correlate with postoperative morbidity and mortality. A prospective study examining staged surgical resection to decrease postoperative mental status decline in patients presenting with a preoperative episode of altered mental status and brainstem compression from a giant pituitary adenoma should also be considered.
References
1. Barzaghi LR, Losa M, Giovanelli M, Mortini P. Complications of transsphenoidal surgery in patients with pituitary adenoma: Experience at a single centre. Acta Neurochir (Wien). 2007. 149: 877-85
2. Garibi J, Pomposo I, Villar G, Gaztambide S. Giant pituitary adenomas: Clinical characteristics and surgical results. Br J Neurosurg. 2002. 16: 133-9
3. Goel A, Nadkarni T. Surgical management of giant pituitary tumours a review of 30 cases. Acta Neurochir (Wien). 1996. 138: 1042-9
4. Guo F, Song L, Bai J, Zhao P, Sun H, Liu X. Successful treatment for giant pituitary adenomas through diverse transcranial approaches in a series of 15 consecutive patients. Clin Neurol Neurosurg. 2012. 114: 885-90
5. Ho RW, Huang HM, Ho JT. The influence of pituitary adenoma size on vision and visual outcomes after trans-sphenoidal adenectomy: A report of 78 cases. J Korean Neurosurg Soc. 2015. 57: 23-31
6. Juraschka K, Khan OH, Godoy BL, Monsalves E, Kilian A, Krischek B. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: Institutional experience and predictors of extent of resection. J Neurosurg. 2014. 121: 75-83
7. Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Paluzzi A, Wang EW, Snyderman CH. Endoscopic endonasal surgery for giant pituitary adenomas: Advantages and limitations. J Neurosurg. 2013. 118: 621-31
8. Leung GK, Law HY, Hung KN, Fan YW, Lui WM. Combined simultaneous transcranial and transsphenoidal resection of large-to-giant pituitary adenomas. Acta Neurochir (Wien). 2011. 153: 1401-8
9. Liu J, Li C, Xiao Q, Gan C, Chen X, Sun W. Comparison of pituitary adenomas in elderly and younger adults: Clinical characteristics, surgical outcomes, and prognosis. J Am Geriatr Soc. 2015. 63: 1924-30
10. Mortini P, Barzaghi R, Losa M, Boari N, Giovanelli M. Surgical treatment of giant pituitary adenomas: Strategies and results in a series of 95 consecutive patients. Neurosurgery. 2007. 60: 993-1002
11. Nakao N, Itakura T. Surgical outcome of the endoscopic endonasalapproach for non-functioning giant pituitary adenoma. J ClinNeurosci. 2011. 18: 71-5
12. Raikundalia MD, Pines MJ, Svider PF, Baredes S, Folbe AJ, Liu JK. Characterization of transsphenoidal complications in patients with acromegaly: An analysis of inpatient data in the united states from 2002 to 2010. Int Forum Allergy Rhinol. 2015. 5: 417-22
13. Zhang X, Li A, Yi S, Zhang Z, Fei Z, Zhang J. Transsphenoidal microsurgical removal of large pituitary adenomas. Chin Med J (Engl). 1998. 111: 963-7