- Department of Surgical Specialties, Neurosurgery Teaching and Assistance Unit, Pedro Ernesto University Hospital, Rio de Janeiro State University, Rio de Janeiro, RJ, Brazil
- Department of Physiological Sciences, Roberto Alcântara Gomes Biology Institute, Rio de Janeiro State University, Rio de Janeiro, RJ, Brazil
- Nervous System Electric Stimulation Laboratory (LabEEL) - Neurosurgery Teaching and Assistance Unit, Pedro Ernesto University Hospital, Rio de Janeiro State University, Rio de Janeiro, RJ, Brazil
Correspondence Address:
Pedro Henrique da C. F. Pinto
Department of Physiological Sciences, Roberto Alcântara Gomes Biology Institute, Rio de Janeiro State University, Rio de Janeiro, RJ, Brazil
Nervous System Electric Stimulation Laboratory (LabEEL) - Neurosurgery Teaching and Assistance Unit, Pedro Ernesto University Hospital, Rio de Janeiro State University, Rio de Janeiro, RJ, Brazil
DOI:10.4103/2152-7806.193926
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pedro Henrique da C. F. Pinto, Flavio Nigri, Gabriel N. Gobbi, Egas M. Caparelli-Daquer. Conversion technique from neuroendoscopy to microsurgery in ventricular tumors: Technical note. 11-Nov-2016;7:
How to cite this URL: Pedro Henrique da C. F. Pinto, Flavio Nigri, Gabriel N. Gobbi, Egas M. Caparelli-Daquer. Conversion technique from neuroendoscopy to microsurgery in ventricular tumors: Technical note. 11-Nov-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/conversion-technique-neuroendoscopy-microsurgery-ventricular-tumors-technical-note/
Abstract
Background:Ventricular tumors represent a major neurosurgical challenge, making endoscopic approach an invaluable tool as it gained importance due to technological advances. Nevertheless, the method is not exempt of risk and limitations, sometimes requiring an open surgery. Thus, initial measurements must be adopted in order to simplify an eventual need for conversion to open craniotomy.
Methods:Here, we describe a series of 6 patients with ventricular tumors approached by neuroendoscopy where the conversion to microsurgery turned out to be necessary. Patients’ average age was 59.5 years (39–75 years). Average tumoral size was 17.8 mm (15–21 mm). There were 2 cases of lateral ventricle subependymoma and 4 cases of third ventricle colloid cysts. A standard surgical incision was performed in the coronal direction, allowing lateral expansion to 10 cm. Moreover, the endoscopic burr hole was enlarged to a 5 cm craniotomy. A small enlargement of the endoscopic cortical access was performed to gain a transcortical microsurgical corridor to the ventricular cavity. The need for conversion arose due to high consistency of the tumor (3 cases), technical problems (2 cases), and cortical collapse (1 case).
Results:There was one case of cerebrospinal fluid fistula and infection and one case of transitory memory disturbance. In both the cases, we obtained a complete functional recovery. Clinical and radiological follow-up showed total tumor removal with no recurrences.
Conclusions:The technique herein described was easy to perform, promptly bypassed the endoscopic limitations, and gathered excellent surgical results. The possibility of adapting the method to other tumor locations may be considered.
Keywords: Conversion technique, microsurgery, neuroendoscopy, open craniotomy, ventricular tumors
INTRODUCTION
Ventricular tumors comprise a variety of benign and malignant mass lesions located within the ventricular cavity or arising from neural structures forming the ventricular system. These tumors are rare comprising less than 1% of all intracranial lesions.[
MATERIALS AND METHODS
Patients
From July 1995 to August 2015, 103 patients with ventricular tumors underwent endoscopic surgery for different purposes, e.g., hydrocephalus, biopsy, septostomy, and tumor resection. In 6 patients, the procedure was converted to open craniotomy. There were 4 men and 2 women, ranging in age from 39 to 71 years (mean, 58.83 years). Clinical summary is presented in
Operative technique
All patients followed the same surgical protocol of supine position, head 45° flexed. The entry point was chosen depending on the tumor side and location. For colloid cysts, the entry point was placed 4–5 cm anterior to the coronal suture and 3 cm lateral to the midline. For tumors located in the frontal horn of lateral ventricle, the Kocher's point was chosen [
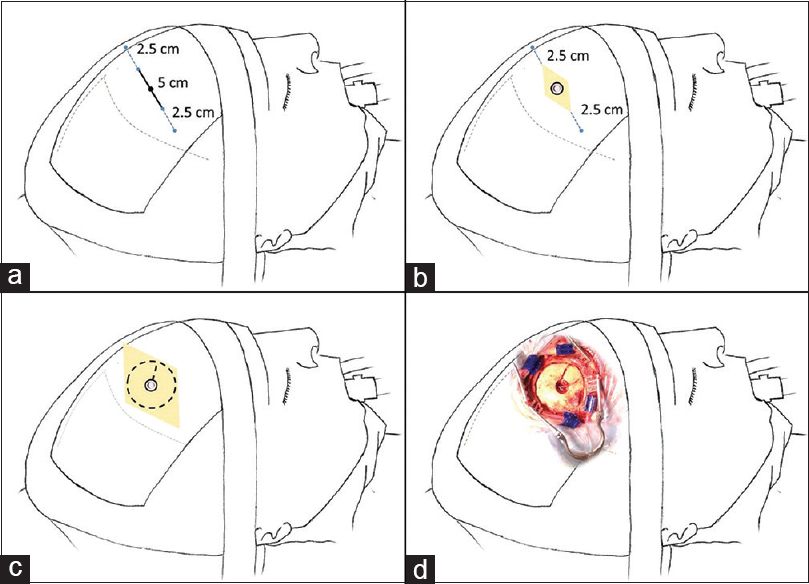
Figure 1
Description of the conversion technique. Patient in the supine position 45° angle head flexed. (a) 5 cm skin mark parallel to the coronal suture and an additional extension of 2.5 cm to each side to be prepared for conversion. (b) 5 cm skin incision and a burr hole to perform neuroendoscopy. (c) Expansion of the incision and a 5 cm diameter craniotomy extending from the endoscopic burr hole to perform microsurgery. (d) Operative aspect of incision and craniotomy
RESULTS
Total tumor removal was obtained in all patients as illustrated in case 3 [
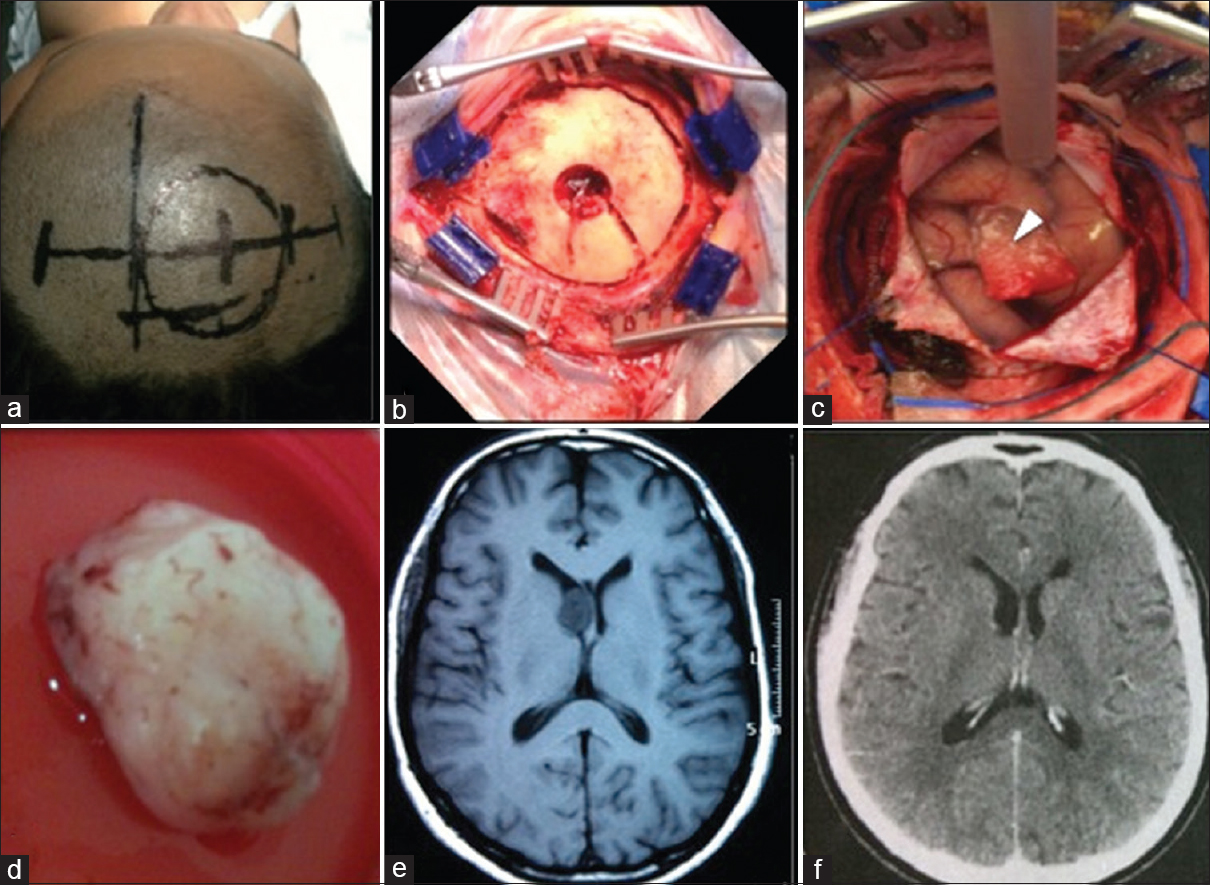
Figure 2
Case 3. (a) Routine surgical planning skin mark. (b) The craniotomy flap of 5 cm diameter. (c) Durotomy with exposure of the cortex and the hemostatic sponge sealing the endoscopic cortical access (arrowhead). Anterior positioning of the cerebral spatula fixed by the Layla brace for microsurgical exploration through the endoscopic path. (d) Subependymoma removed after conversion. (e) Axial T1-weighted magnetic resonance imaging obtained 2 months preoperative. (f) Axial computed tomography of the head obtained 3 months after the surgery
DISCUSSION
Microsurgical resection is the standard technique for ventricular tumor treatment.[
Applied by Dandy[
Considering that the endoscopic approach has the same cortical trajectory as the transcortical transventricular approach, the conversion is made just extending the size of the craniotomy. Instead of the classic incision parallel to the sagittal suture, commonly performed in neuroendoscopy, an incision parallel to the coronal suture is chosen. This allowed extending the incision without passing the anterior hairline in cases of conversion to open craniotomy in all patients of this series. There are no data in the literature defining the best conversion technique, however, the operative technique applied in our patients was satisfactory, easy, and fast to perform. Although simple and probably a logical choice for most neurosurgeons, here, we provide evidence that this surgical option is safe and effective.
CONCLUSIONS
The standard conversion technique described in this series was easy to perform, showed excellent clinical and radiological results, bypassed endoscopic limitations, and made possible complete resection of ventricular lesions in all patients. There is little information about conversion technique in the literature, and therefore validation through further studies on the subject is necessary. The possibility of adapting the method according to ventricle locations other than the anterior horn of the lateral ventricle and the third ventricle roof may be considered.
Financial support and sponsorship
Authors received financial support from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Diego Ramon Nigri for his assistance in preparing and drawing the figures.
References
1. Abdou MS, Cohen AR. Endoscopic treatment of colloid cysts of the third ventricle. J Neurosurg. 1998. 89: 1062-8
2. Ahmad F, Sandberg DI. Endoscopic management of intraventricular brain tumors in pediatric patients: A review of indications, techniques, and outcomes. J Child Neurol. 2010. 25: 359-67
3. Apuzzo MLJ, Chikovani OK, Gott PS, Teng EL, Zee CS, Giannotta SL. Transcalossal, interfornicial approaches for lesions affecting the third ventricle: Surgical considerations and consequences. Neurosurgery. 1982. 10: 547-54
4. Barber SM, Rangel-Castilla L, Baskin D. Neuroendoscopic resection of intraventricular tumors: A systematic outcomes analysis. Minim Invasive Surg 2013. 2013. p. 898753-
5. Bertalanffy H, Krayenbuhl N, Wess C, Bozinov O, Winn HR.editors. Ventricular Tumors. Youmans Neurological Surgery. Philadelfia, PA: WB Saunders; 2011. p. 1534-5
6. Cappabianca P, Cinalli G, Gangemi M, Brunori A, Cavallo LM, Divitiis E. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008. 62: 575-97
7. Chamoun R, Couldwell WT. Transcortical-transforaminal microscopic approach for purely intraventricular craniopharyngioma. Neurosurg Focus. 2013. 34:
8. Chowdhry SA, Cohen AR. Intraventricular neuroendoscopy: Complication avoidance and management. World Neurosurg. 2013. 79: S15.e1-10
9. Cohen-Gadol AA. Minitubular transcortical microsurgical approach for gross total resection of third ventricular colloid cysts: Technique and assessment. World Neurosurg. 2013. 79: 207.e7-10
10. Dandy W. Cerebral Ventriculoscopy. Bull Johns Hopkins Hosp. 1922. 33: 189-90
11. Davis L.editorsNeurological Surgery. Philadelphia, PA: Lea & Febiger; 1936. 1:
12. Decq P, Le Guerinel C, Brugières P, Djindjian M, Silva D, Kéravel Y. Endoscopic Management of Colloid Cysts. Neurosurgery. 1998. 42: 1288-94
13. Enchev Y, Oi S. Historical trends of neuroendoscopic surgical techniques in the treatment of hydrocephalus. Neurosurg Rev. 2008. 31: 249-61
14. Gaab MR, Schroeder HW. Neuroendoscopic approach to intraventricular lesions. J Neurosurg. 1998. 88: 496-505
15. Gore PA, Nakaji P, Deshmukh V, Rekate HL. Synchronous endoscopy and microsurgery: A novel strategy to approach complex ventricular lesions. J Neurosurg Pediatr. 2006. 105: 485-9
16. Greenlee JD, Teo C, Ghahreman A, Kwok B. Purely endoscopic resection of colloid cysts. Neurosurgery. 2008. 62: 51-5
17. Grondin RT, Hader W, MacRae ME, Hamilton MG. Endoscopic versus microsurgical resection of third ventricle colloid cysts. Can J Neurol Sci. 2007. 34: 197-207
18. McLaughlin N, Prevedello DM, Engh J, Kelly DF, Kassam AB. Endoneurosurgical resection of intraventricular and intraparenchymal lesions using the port technique. World Neurosurg. 2013. 79: S18.e1-8
19. Patel P, Cohen-Gadol AA, Boop F, Klimo P. Technical strategies for the transcallosal transforaminal approach to third ventricle tumors: Expanding the operative corridor. J Neurosurg Pediatr. 2014. 14: 365-71
20. Peltier J, Roussel M, Gerard Y, Lassonde M, Deramond H, Le Gars D. Functional consequences of a section of the anterior part of the body of the corpus callosum: Evidence from an interhemispheric transcallosal approach. J Neurol. 2012. 259: 1860-7
21. Schroeder HWS. General principles and intraventricular neuroendoscopy: Endoscopic Techniques. World Neurosurg. 2013. 79: S14.e23-28
22. Schroeder HW. Intraventricular tumors. World Neurosurg. 2013. 79: S17.e15-9
23. Symss NP, Ramamurthi R, Rao SM, Vasudevan MC, Jain PK, Pande A. Management outcome of the transcallosal, transforaminal approach to colloid cysts of the anterior third ventricle: An analysis of 78 cases. Neurol India. 2011. 59: 542-7
24. Teo C, Nakaji P. Neuro-oncologic applications of endoscopy. Neurosurg Clin N Am. 2004. 15: 89-103
25. Yasargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. 2008. 62: 1029-40