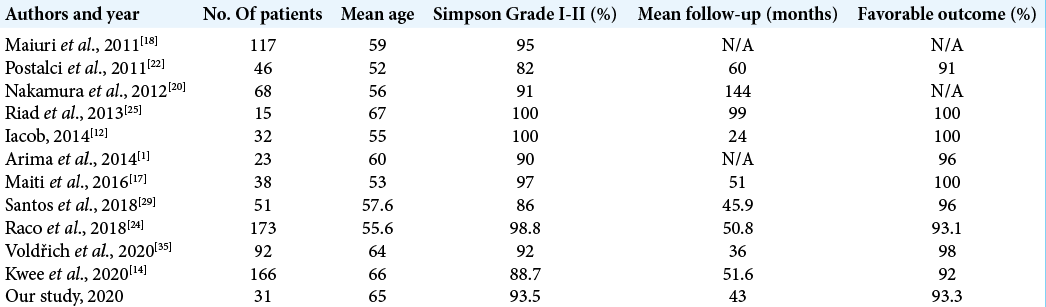- Department of Neurosurgery, Faculty of Medicine, Medical University of Plovdiv, Plovdiv, Bulgaria,
- Clinic of Neurosurgery, Sv. Georgi University Hospital, Plovdiv, Bulgaria,
- Department of Health Management and Health Economics, Faculty of Public Health, Medical University of Plovdiv, Plovdiv, Bulgaria.
DOI:10.25259/SNI_927_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Atanas Davarski1, Borislav Kitov1, Georgi Apostolov2, Ivo Kehayov1, Rumyana Stoyanova3. Correlations between preoperative clinical factors and treatment outcome of spinal meningiomas – A retrospective study of a series of 31 cases. 25-May-2021;12:236
How to cite this URL: Atanas Davarski1, Borislav Kitov1, Georgi Apostolov2, Ivo Kehayov1, Rumyana Stoyanova3. Correlations between preoperative clinical factors and treatment outcome of spinal meningiomas – A retrospective study of a series of 31 cases. 25-May-2021;12:236. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10824
Abstract
Background: The purpose of the current study is to identify the correlations between the most important preoperative clinical factors and the outcome of surgery of spinal meningiomas (SM).
Methods: We performed a retrospective analysis of the medical history, clinical, paraclinical, neuroimaging, and surgical protocol data in 31 patients with SM who underwent surgical resection at our institution from January 2011 to July 2020. The degree of resection was assessed on the Simpson scale. The modified McCormick scale was used to monitor the effect and outcome of treatment at admission, discharge, and at further follow-up.
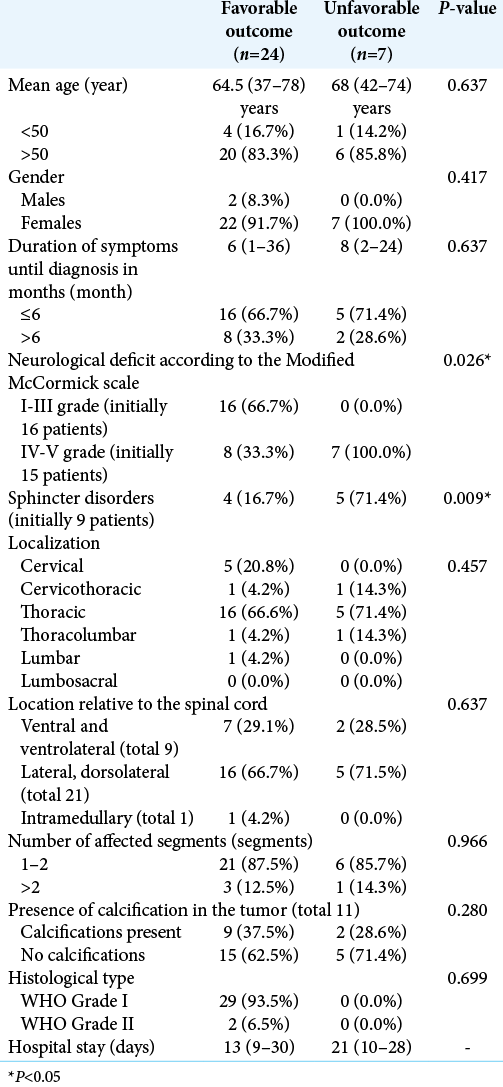
Results: The average age of the patients was 65 years (37-78). Vertebral pain and motor deficits were the most common initial symptoms that occurred in 26 (89.6%) and 29 (93.5%) patients, respectively. Sphincter disorders were found in 9 (29%) patients. Total resection (Simpson Grade I – II) was achieved in 29 patients (93.5%). We achieved a favorable outcome (McCormick Gr. I to III) in 93.3% of patients. The degree of the neurological deficit (P = 0.026) and the presence of sphincter disorders (P = 0.009) were the preoperative clinical factors that most significantly correlated with the outcome of treatment.
Conclusion: The outcome from the surgical treatment of SM correlated significantly with the degree of the preoperative neurological deficit. Therefore, patients presenting with more severe symptoms are expected to have worse outcomes.
Keywords: Myelopathy, Outcome, Risk factors, Spinal meningioma, Surgery
INTRODUCTION
Spinal meningiomas (SM) represent about 1–2% of all meningiomas and 25–45% of all intradural extramedullary spinal tumors.[
The upper cervical spine and foramen magnum are also common sites for SM occurrence.[
The purpose of the current study is to identify the correlations between the most important preoperative clinical factors and the outcome of surgery of SM.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of St Georgi University Hospital. The patient data from medical histories, clinical presentation, paraclinical tests, diagnostic imaging, and surgical protocols were retrospectively collected. Attention was paid to the symptoms at onset, clinical presentation at admission, neuroimaging data, localization, type of surgical intervention, histology, and outcome of the disease. The degree of resection was assessed on the Simpson scale.[
Statistical analysis
The Chi-square test was used to compare categorical data and the nonparametric Mann–Whitney U test to compare variables grouped in rank scales. The bilateral significance level was determined to be P < 0.05.
RESULTS
Thirty-one patients underwent 32 surgical interventions for SM at our institution from January 2011 to July 2020 (29 women [93.5%] and 2 men [6.5%] aged between 37 and 78 years, mean age 65 years). One patient underwent surgery for meningioma recurrence 4 years after the first operation. Twenty-eight (90.3%) of the tumors were intradural extramedullary, two (6.5%) had extradural extension, and one (3.2%) was intramedullary.
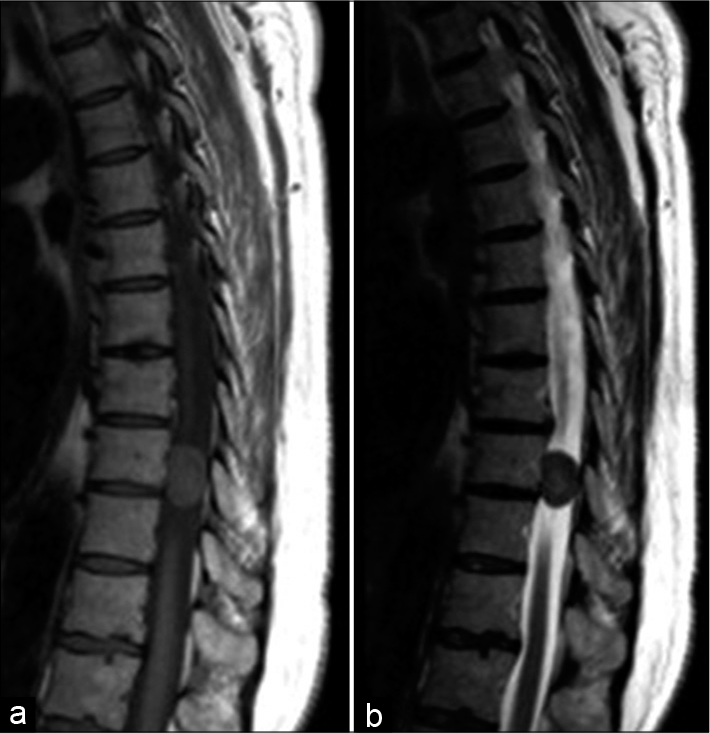
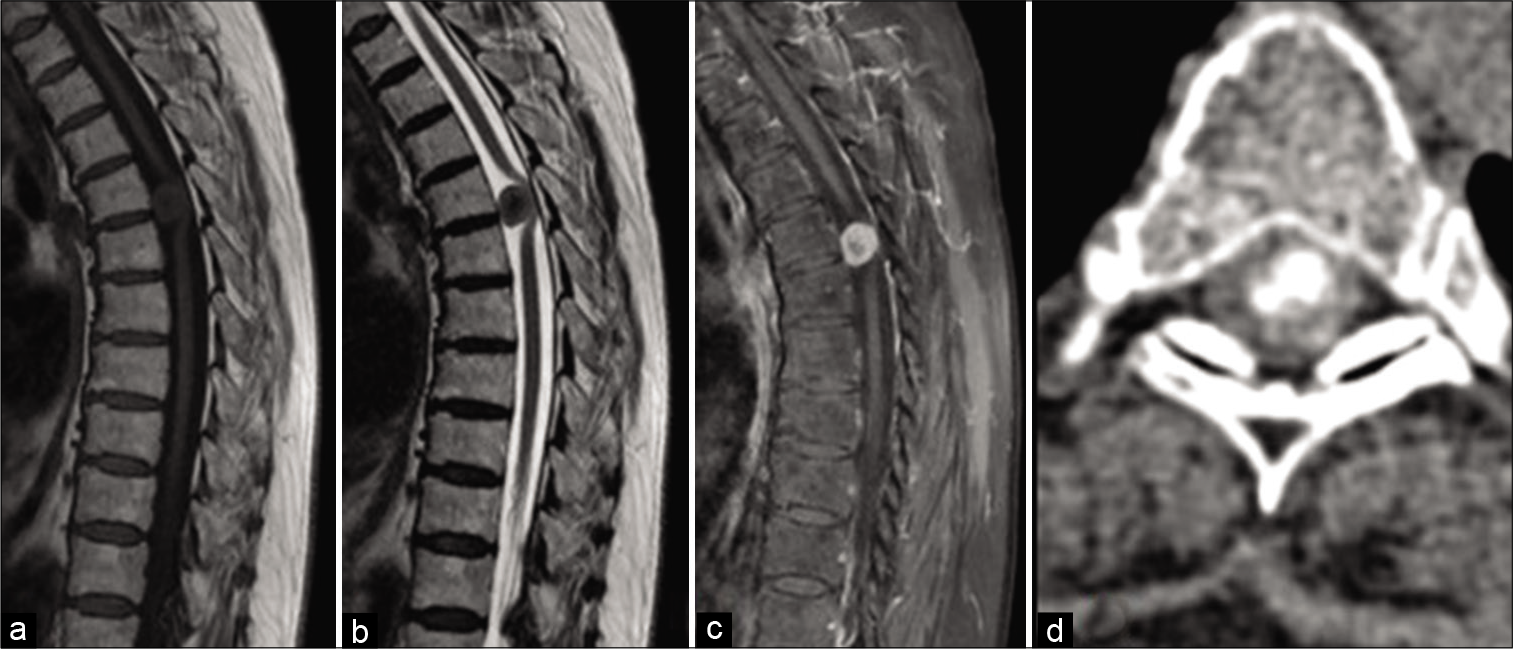
In 26 (83.9%) patients, the disease debuted with low back and/or back pain, and 29 (93.5%) patients had additional motor deficits that gradually progressed over time. Twenty-one of the patients (67.7%) had lower extremity paraparesis, 3 (9.7%) had lower extremity paraplegia, 4 (12.9%) had quadriparesis, and one (3.2%) had unilateral lower extremity paresis. Sphincter disorders were found in 9 (29%) patients. The period from the disease onset to its diagnosis varied from 1 to 36 months (median 6.0 months; IQR = 8.0). The clinical symptoms, the localization, and SM’s location relative to the axial section of the spinal cord and cauda equina are presented in [
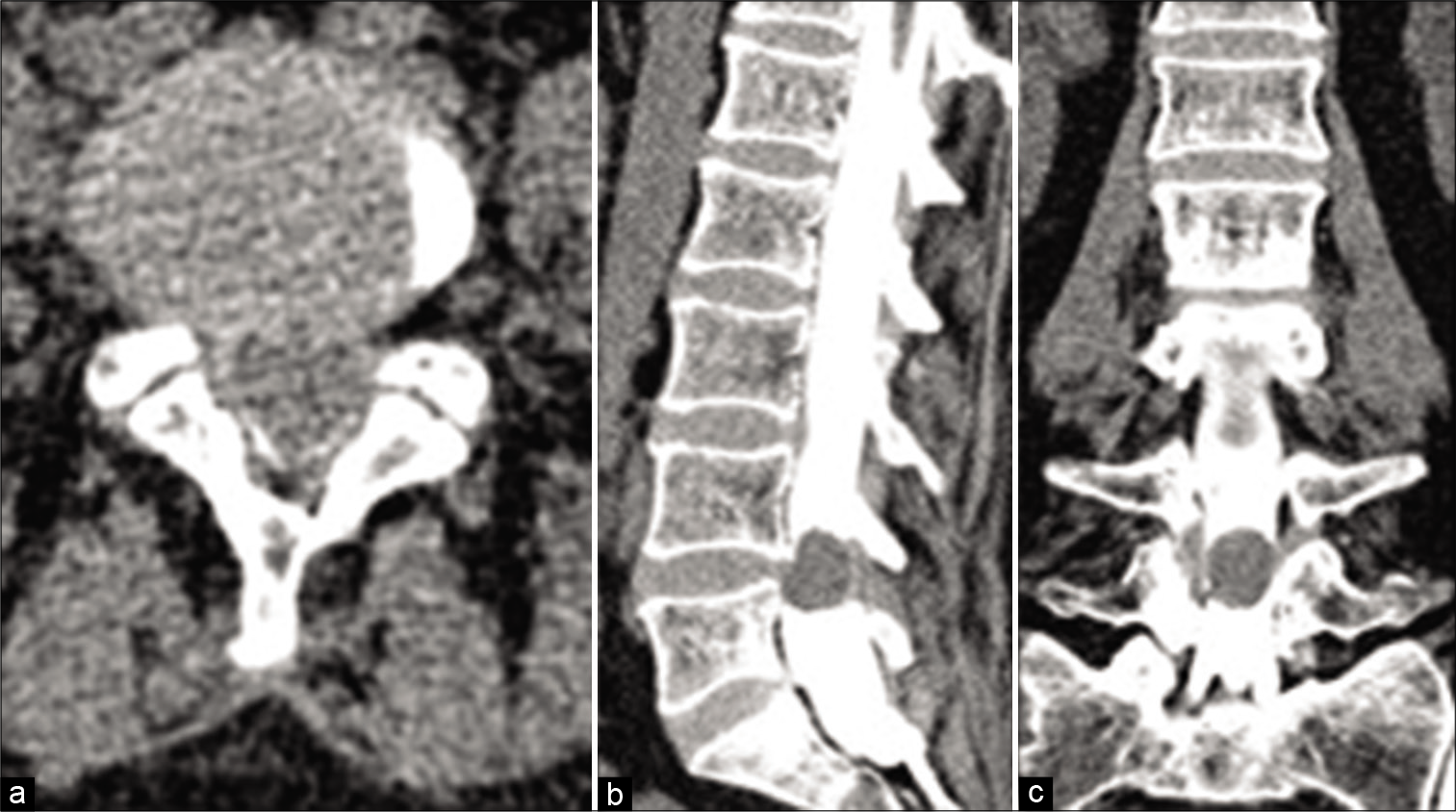
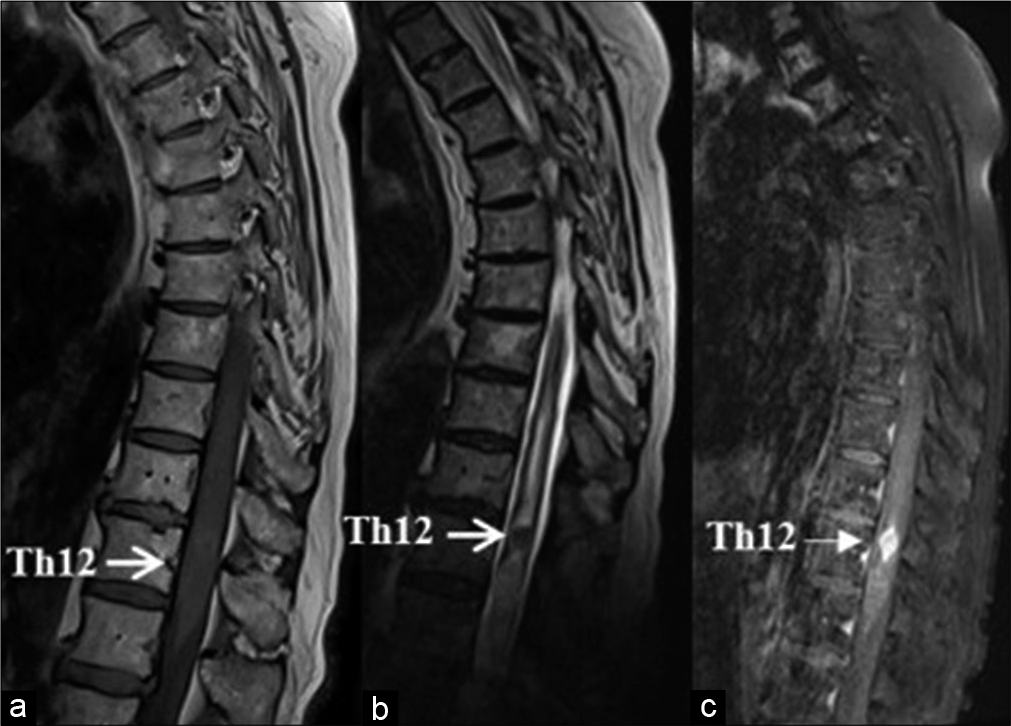
In 1 patient who was contraindicated to magnetic resonance imaging (MRI) investigation, the diagnosis was achieved by computed tomography (CT)-assisted myelography [
Figure 4:
MRI and CT of a patient with ventrolateral meningioma at the level of T5. (a) Sagittal T1 MRI image – the tumor elicits isointense signal; (b) sagittal T2 MRI image – a central area with a more pronounced hypointense “dark” signal is visualized that is consistent with intratumoral calcification; (c) sagittal T1 MRI image with gadolinium enhancement – lack of accumulation of the contrast in the central calcified area; (d) axial CT – the presence of calcifications is well visible.
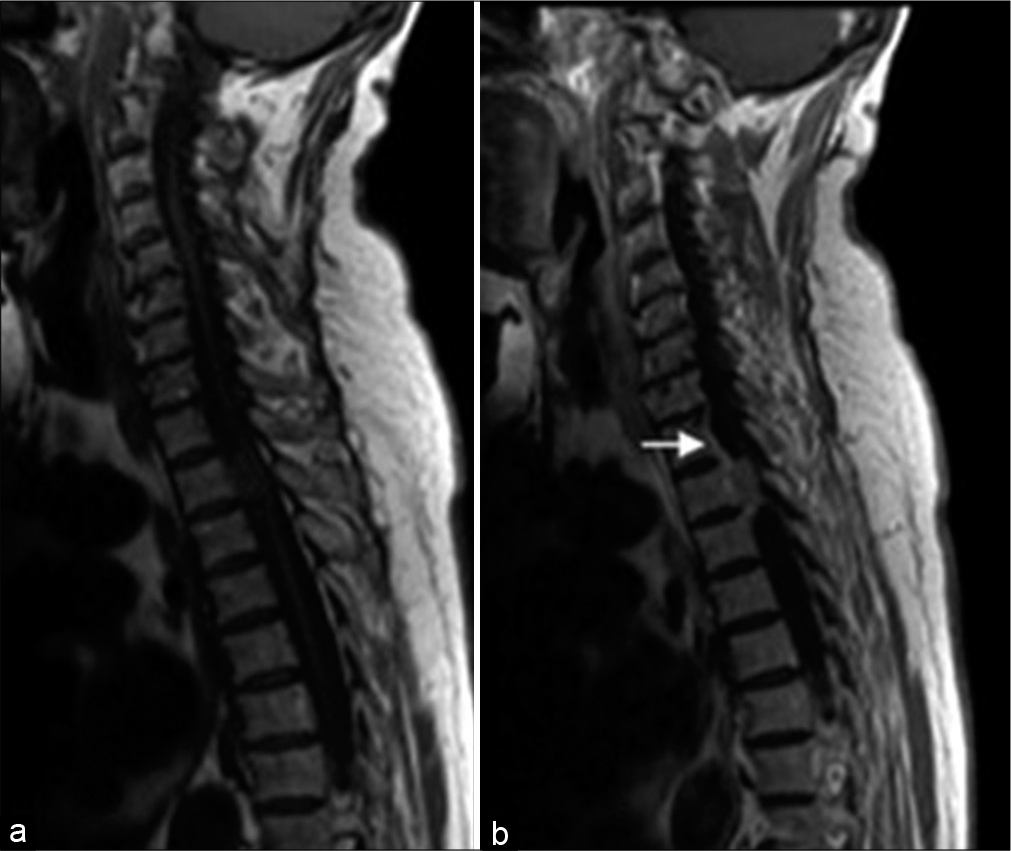
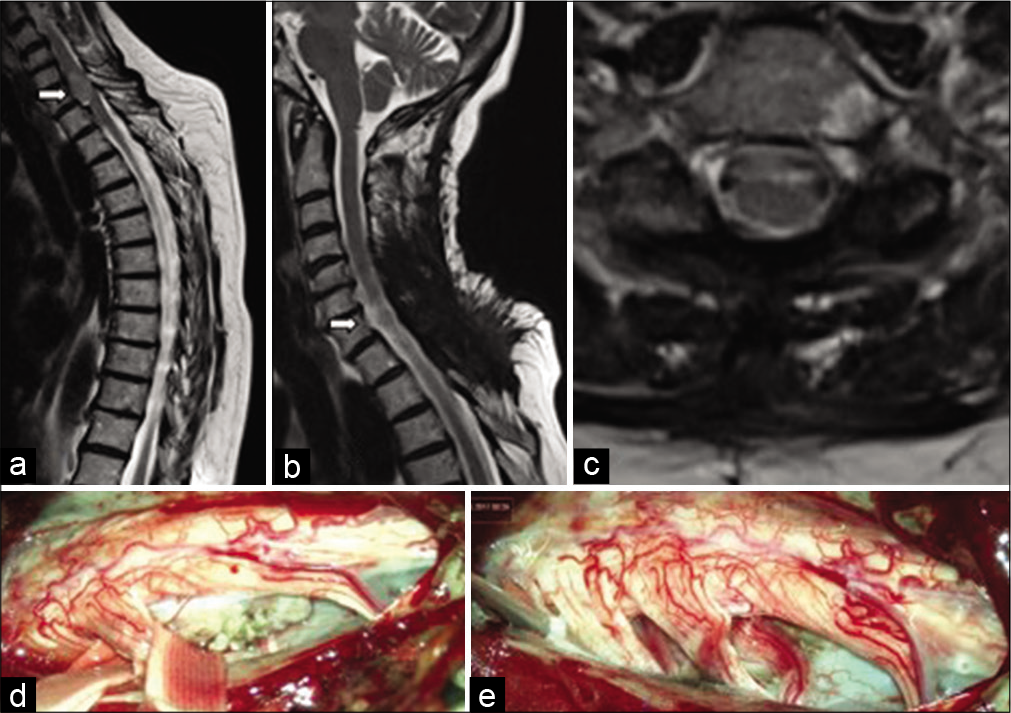
Figure 5:
Sagittal MRI of a patient with an intramedullary meningioma (white arrow) (a) T1-weighted image shows an isointense lesion with a spindle-shaped expansion of the medulla; (b) T2-weighted image demonstrates ovoid hypointense lesion with associated hydromyelia; (c) Enhanced T1-weighted image shows a homogeneous accumulation of the contrast media by the tumor.
We used posterior or posterolateral surgical access in all operated patients. The goal of surgery was total tumor removal. In 30 patients (96.8%), the access used was laminectomy and in one – hemilaminectomy. Total resection (Simpson Grade I and II) was achieved in 29 patients (93.5%) [
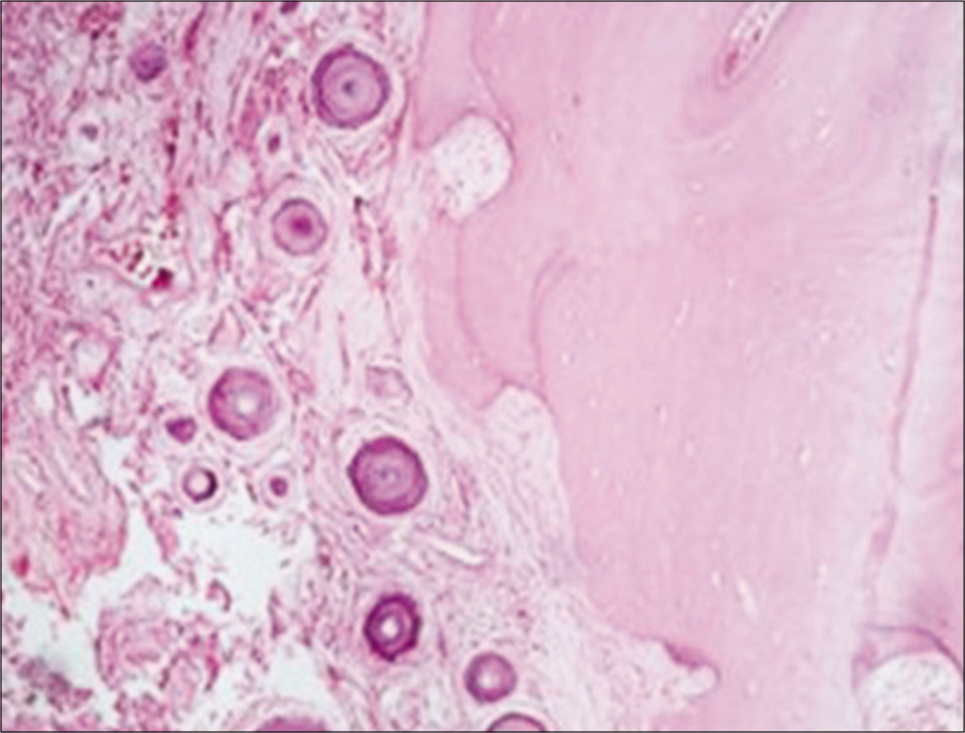
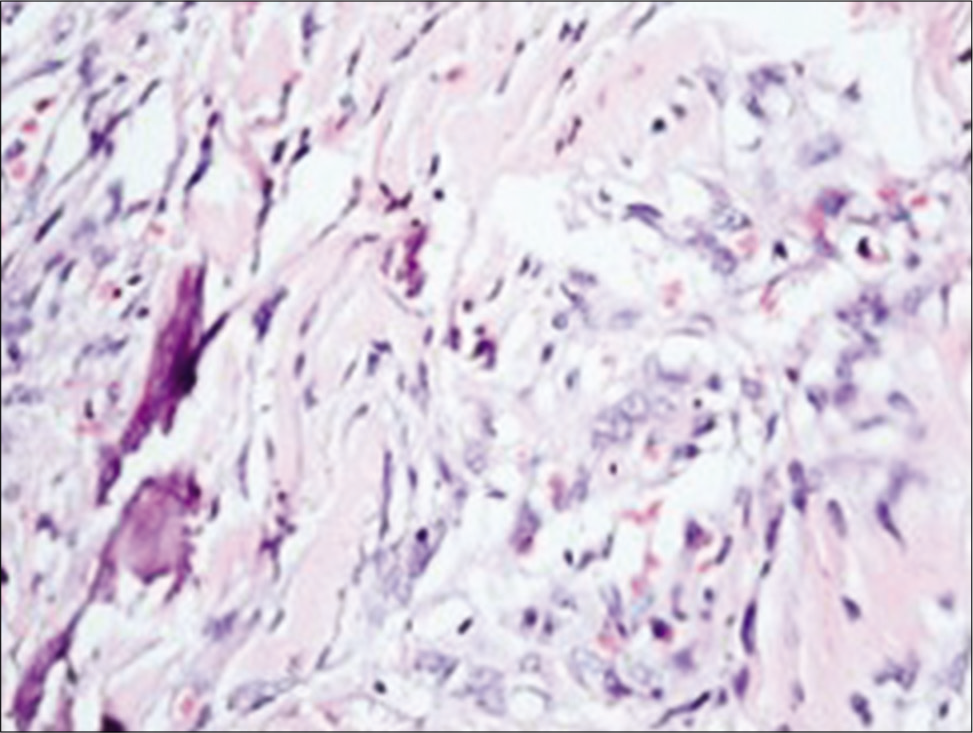
The histological result in 29 patients revealed WHO Grade I psammomatous type of meningioma. In one patient, the tumor had pronounced adjacent bone metaplasia [
In 30 patients (96.8%), the postoperative period was uneventful. All patients had improvement in their neurological status. One patient developed hemorrhagic stroke at the second postoperative day, which spontaneously resorbed after conservative treatment for 17 days. The median hospital stay was 14.5 days (Min = 9.0; Max = 30.0; IQR = 10.25).
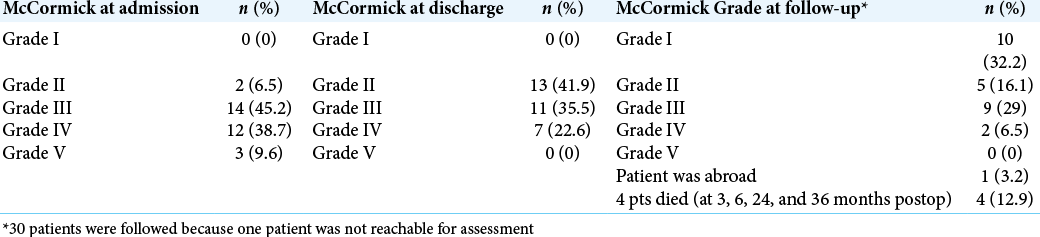
Preoperatively, the patients with McCormick Grade I-III were 16 (51.6%), while the remaining 15 (48.4%) were with McCormick Grade IV-V. After treatment, the number of patients with McCormick Grade I-III increased to 24 (77.4%), while those with McCormick Grade IV-V decreased to 7 (22.6%), and during follow-up, their number further decreased to 2 (6.4%). At the time of admission and discharge, none of the patients had an intact neurological function and normal gait. At follow-up, there were 10 such patients (32.2%) [
The statistical analysis found that only the degree of preoperative neurological deficit (P = 0.026) and the presence of sphincter disorders (P = 0.009) were significantly correlated to the outcome after treatment [
DISCUSSION
SMs can be found along the entire spine axis, but primarily in the thoracic region and have a frequency of 0.5–2 per 100,000 people per year.[
SMs with an intradural and extradural spread are observed in 5–6% of cases, while the intramedullary and purely epidural variants are extremely rare.[
Our series confirms that SMs can be observed in all age groups. Twenty-three of the patients (79.3%) were between the 5th and 7th decade of their life, with 15 (51.7%) of them being older than 65 years. Due to the influence of specific sex hormones in tumorogenesis, meningiomas are much more common in women, with a female-to-male ratio ranging from 4:1 to 10:1.[
In general, SMs are manifested clinically by symptoms of progressive radiculopathy and/or myelopathy.[
While IONM for intramedullary tumors has become a standard in neurosurgical practice, IONM for intradural extramedullary tumors is still under debate.[
Good or excellent outcome can be achieved in 79% to 98% of cases.[
Previous studies have found that factors such as ventral or ventrolateral axial tumor location, large tumor size, T2 cord signal changes, the presence of extensive calcifications, prolonged presentation before diagnosis, WHO grade > I, Simpson resection grade II, or higher can negatively affect the outcome because are associated with an increased risk of long-term morbidity.[
Despite the retrospective design, a relatively small number of patients and the data that were not statistically analyzed through multivariate analysis, this study found that the outcome of SM treatment was significantly correlated to the degree of the preoperative neurological deficit and the presence of sphincter disorders. A potential discrepancy in the current study is the fact that it demonstrated that more severe preoperative symptoms were associated with the poorer outcome while the length of time with symptoms had no significant effect on outcome.
CONCLUSION
Spinal surgeons should be aware of the insidious natural evolution of SM. Despite being benign lesions, SM can lead to permanent disability if left untreated. The outcome of surgical treatment of SMs is strongly correlated to the severity of the preoperative neurological deficit. Therefore, patients presenting with more severe symptoms are expected to have worse outcomes. However, further prospective studies, which include larger number of patients, are needed to elucidate the identified correlations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arima H, Takami T, Yamagata T, Naito K, Abe J, Shimokawa N. Surgical management of spinal meningiomas: A retrospective case analysis based on preoperative surgical grade. Surg Neurol Int. 2014. 5: 333-9
2. Bloomer CW, Ackerman A, Bhatia AG. Imaging for spine tumors and new applications. Top Magn Reson Imaging. 2006. 17: 69-87
3. Caroli E, Acqui M, Roperto R, Ferrante L, D’Andrea G. Spinal en plaque meningiomas: a contemporary experience. Neurosurgery. 2004. 55: 1275-9
4. Cheng C, Wang J, Zhao S, Tao B, Bai S, Shang A. Intramedullary thoracic meningioma: A rare case report and review of the literature. World Neurosurg. 2019. 129: 176-80
5. Cofano F, Giambra C, Costa P, Zeppa P, Bianconi A, Mammi M. Management of extramedullary intradural spinal tumors: The impact of clinical status, intraoperative neurophysiological monitoring and surgical approach on outcomes in a 12-year double-center experience. Front Neurol. 2020. 11: 598619
6. Gezen F, Kahraman S, Canakci Z, Beduk A. Review of 36 cases of spinal cord meningioma. Spine. 2000. 25: 727-31
7. Ghadirpour R, Nasi D, Iaccarino C, Giraldi D, Sabadini R, Motti L. Intraoperative neurophysiological monitoring for intradural extramedullary tumors: Why not?. Clin Neurol Neurosurg. 2015. 130: 140-9
8. Ghaffari-Rafi A, Mehdizadeh R, Ghaffari-Rafi S, Leon-Rojas J. Demographic and socioeconomic disparities of benign and malignant spinal meningiomas in the United States. Neurochirurgie. 2021. 67: 112-8
9. Gilard V, Goia A, Ferracci FX, Marguet F, Magne N, Langlois O. Spinal meningioma and factors predictive of post-operative deterioration. J Neurooncol. 2018. 140: 49-54
10. Harel R, Schleifer D, Appel S, Attia M, Cohen ZR, Knoller N. Spinal intradural extramedullary tumors: The value of intraoperative neurophysiologic monitoring on surgical outcome. Neurosurg Rev. 2017. 40: 613-9
11. Hohenberger C, Gugg C, Schmidt NO, Zeman F, Schebesch KM. Functional outcome after surgical treatment of spinal meningioma. J Clin Neurosci. 2020. 77: 62-6
12. Iacob G. Spinal meningiomas. Personal experience and review of literature. Rom Neurosurg. 2014. 21: 146-60
13. Kleklamp J, Samii M.editors. Extramedullary tumors. Surgery of Spinal Tumors. Berlin, Heidelberg: Springer-Verlag; 2007. p. 248-60
14. Kwee LE, Harhangi BS, Ponne GA, Kros JM, Dirven CM, Dammers R. Spinal meningiomas: Treatment outcome and long-term follow-up. Clin Neurol Neurosurg. 2020. 198: 106238
15. Levy WJ, Bay J, Dohn D. Spinal cord meningioma. J Neurosurg. 1982. 57: 804-12
16. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
17. Maiti TK, Bir SC, Patra DP, Kalakoti P, Guthikonda B, Nanda A. Spinal meningiomas: clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. 2016. 41: E6
18. Maiuri F, De Caro ML, de Divitiis O, Vergara P, Mariniello G. Spinal meningiomas: Age-related features. Clin Neurol Neurosurg. 2011. 113: 34-8
19. McCormick PC, Post KD, Stein BM. Intradural extramedullary tumors in adults. Neurosurg Clin N Am. 1990. 1: 591-608
20. Nakamura M, Tsuji O, Fujiyoshi K, Hosogane N, Watanabe K, Tsuji T. Long-term surgical outcomes of spinal meningiomas. Spine (Phila Pa 1976). 2012. 37: E617-23
21. Pereira CU, Dias LA, Dezena RA. Spinal Meningiomas: Report of 14 cases and literature review. J Bras Neurocirurg. 2015. 26: 110-5
22. Postalci L, Tugcu B, Gungor A, Guclu G. Spinal meningiomas: Recurrence in ventrally located individuals on long-term follow-up; a review of 46 operated cases. Turk Neurosurg. 2011. 21: 449-53
23. Raco A, Pesce A, Miscusi M, Signorelli F.editors. Surgical treatment of spinal meningiomas. From Bench to Bedside Trauma, Tumors, Spine, Functional Neurosurgery. London: InTechOpen; 2016. p. 99-110
24. Raco A, Pesce A, Toccaceli G, Domenicucci M, Miscusi M, Delfini R. Factors leading to a poor functional outcome in spinal meningioma surgery: Remarks on 173 cases. Neurosurgery. 2018. 80: 602-9
25. Riad H, Knafo S, Segnarbieux F, Lonjon N. Spinal meningiomas: Surgical outcome and literature review. Neurochirurgie. 2013. 59: 30-4
26. Ruggeri AG, Fazzolari B, Colistra D, Cappelletti M, Marotta N, Delfini R. Calcified spinal meningiomas. World Neurosurg. 2017. 102: 406-12
27. Sahni D, Harrop JS, Kalfas IH, Vaccaro AR, Weingarten D. Exophytic intramedullary meningioma of the cervical spinal cord. J Clin Neurosci. 2008. 15: 1176-9
28. Sandalcioglu IE, Hunold A, Muller O, Stolke D, Asgari S. Spinal meningiomas: Critical review of 131 surgically treated patients. Eur Spine J. 2008. 17: 1035-41
29. Santos RC, de Amoreira Gepp R. Benefits of spinal meningioma resection. Surg Neurol Int. 2019. 9: 16
30. Schwarts TH, McCormick PC, Winn HR.editors. Spinal cord tumors in adults. Youmans Neurological Surgery. Philadelphia, PA: Saunders; 2004. 4: 4817-34
31. Setzer M, Vatter H, Marquardt G, Seifert V, Vrionis FD. Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus. 2007. 23: E14
32. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatr. 1957. 20: 22-39
33. Traul DE, Shaffrey ME, Schiff D. Part I: Spinal-cord neoplasms intradural neoplasms. Lancet Oncol. 2007. 8: 35-45
34. Tsai EC, Butler J, Benzel EC, Lee JH.editors. Spinal meningiomas. Meningiomas. London: Springer; 2009. p. 529-39
35. Voldřich R, Netuka D, Beneš V. Spinal meningiomas: Is Simpson grade II resection radical enough?. Acta Neurochir. 2020. 162: 1401-8
36. Yuan D, Liu D, Yuan XR, Xi J, Ding X. Intramedullary thoracic spinal cord meningioma: A rare case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2013. 74: e136-9