- Department of Neurosurgery, University of Tennessee Health Science Center, Memphis, United States
- Department of Neurosurgery, University of Rochester Medical Center, Rochester, United States
- College of Medicine, University of Tennessee Health Science Center, Memphis, United States
Correspondence Address:
Mustafa Motiwala, Department of Neurosurgery, University of Tennessee Health Science Center, Memphis, United States.
DOI:10.25259/SNI_106_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kara A. Parikh1, Vincent N. Nguyen2, Mustafa Motiwala1, Taylor J. Orr1, Kaan Yagmurlu1, C. Stewart Nichols3, Adam S. Arthur1, Jeffrey M. Sorenson1, L. Madison Michael II1, Nickalus R. Khan1. Cranial-orbital approaches for vascular pathology: A review of surgical approach selection and technical considerations. 23-May-2025;16:191
How to cite this URL: Kara A. Parikh1, Vincent N. Nguyen2, Mustafa Motiwala1, Taylor J. Orr1, Kaan Yagmurlu1, C. Stewart Nichols3, Adam S. Arthur1, Jeffrey M. Sorenson1, L. Madison Michael II1, Nickalus R. Khan1. Cranial-orbital approaches for vascular pathology: A review of surgical approach selection and technical considerations. 23-May-2025;16:191. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13578
Abstract
Background: Modified cranial approaches for vascular pathology are sometimes necessary to enhance exposure and can be tailored by the pathology treated and surgical conditions. The authors outline these approaches, comparing the advantages and disadvantages of each.
Methods: Surgical footage of the senior author performing cranial-orbital skull base approaches for intracranial aneurysms as part of routine care was reviewed to identify and describe the advantages and disadvantages of these approaches to vascular pathology. The variations of cranial-orbital approaches included supraorbital, lateral supraorbital (LSO), orbito-pterional, cranio-orbital, and transcavernous approaches. Four illustrative cases are included. The literature was also reviewed for a concise compilation and summary of technical considerations and comparisons of cranial-orbital approaches for the microsurgical treatment of vascular pathology.
Results: The supraorbital approach provides a trajectory along the orbital roof, allowing access to anterior circulation aneurysms without drilling the anterior clinoid process. While this approach is suited for inferiorly and anteriorly projecting anterior communicating artery (AcomA) aneurysms, orbito-pterional approaches are better suited for superiorly projecting AcomA aneurysms. The LSO approach allows access to anterior circulation and low-lying basilar apex lesions. The orbito-pterional approach is an “outside-in” approach to access the intracranial space from the orbit; the cranio-orbital approach is considered an “inside-out” approach to access the orbit from the intracranial space.
Conclusion: Modifications of the traditional pterional craniotomy are useful for various anterior and posterior circulation vascular pathologies. Extensions of these surgical corridors with transcavernous approaches can also be useful. Understanding the advantages and disadvantages of each is important in optimal approach selection.
Keywords: Cranio-orbital approach, Neurovascular, Orbito-cranial approach, Skull base, Supraorbital, Transcavernous approach
INTRODUCTION
Cranial approaches for vascular pathology often incorporate a skull base osteotomy or approach. The long established pterional – or fronto-temporal – approach was described by Dandy in 1922, and its cranial-orbital variations continue to be a workhorse for approaching intracranial vascular pathology.[
MATERIALS AND METHODS
We identified commonly employed cranial-orbital skull base approaches for treating vascular pathology. The main variations of cranial-orbital approaches included supraorbital, lateral supraorbital (LSO), orbito-pterional, cranio-orbital, and transcavernous approaches. Cases performed by the senior author utilizing these approaches as part of routine care were identified. (Case surgeries are routinely recorded in our practice for later review and to improve surgeon performance.) Surgical footage of representative cases was reviewed to identify and describe the advantages and disadvantages of each approach for specific anatomical pathology and locations. Finally, we reviewed the literature for a concise compilation and summary of previously described technical considerations and comparisons of cranial-orbital approaches for the microsurgical treatment of vascular pathology.
RESULTS
The supraorbital approach provides a trajectory along the orbital roof and access to anterior circulation aneurysms that do not require extensive drilling of the anterior clinoid process. The superior extension of the trajectory is limited by the orbital rim, which can also be removed as needed. This approach is well suited for inferiorly and anteriorly projecting anterior communicating artery (AcomA) aneurysms and select paraclinoid aneurysms.[
The orbital extensions of pterional approaches are better suited for superiorly and posteriorly projecting AcomA aneurysms that often need considerably more dissection in the interhemispheric fissure.[
The LSO approach also allows adequate access to anterior circulation and selects high-positioned basilar bifurcation or basilar-SCA aneurysms.[
A summary of a literature review of the use of these approaches for vascular pathology is shown in
DISCUSSION
Supraorbital approach: General approach selection and technique considerations
The supraorbital craniotomy is often used as a “keyhole” approach, offering a minimally invasive surrogate with little brain exposure and retraction. It allows a trajectory along the orbital roof for the surgeon to access AcomA, ipsilateral supraclinoid internal carotid artery (ICA), posterior communicating artery (PcomA), anterior choroidal artery (AchA), and proximal, middle cerebral artery (MCA) aneurysms arising near the circle of Willis. The orbital rim limits the superior extension of the trajectory. The supraorbital craniotomy does not allow for an extensive superior trajectory of the operative corridor for distal anterior circulation aneurysms, although this corridor can be enhanced with an orbitotomy. A smaller keyhole approach can limit the approach to fewer angles of attack than a larger craniotomy would afford.[
Advantages and disadvantages of surgical targets
Anterior circulation
AcomA
Supraorbital craniotomies can provide an excellent working corridor for AcomA aneurysms, affording more exposure to aneurysms than traditional pterional approaches.[
ICA, PcomA, and AchA
The subfrontal approach allows medial Sylvian fissure dissection, achieving frontal lobe retraction without temporal lobe retraction that gives visualization of the ICA bifurcation.[
MCA
To achieve optimal exposure and visualization for MCA aneurysms, the supraorbital craniotomy may be extended laterally several millimeters. Sylvian fissure dissection, as described above, allows access to the aneurysm, although aneurysms distal to the genu are not easily accessed from this approach.[
Posterior circulation
Less commonly, basilar apex aneurysms can be accessed from this approach. It is important to take note of the height of the basilar apex in relation to the anterior skull base. Some authors suggest that basilar apex aneurysms within 1.5 cm of the horizontal line of the anterior skull base are accessible,[
Regardless of the aneurysm location, the supraorbital approach is less suited for aneurysms of larger or complex morphology or multiple aneurysms that require multiple angles of clipping compared to larger craniotomies that allow broader angles of approach. In patients where a significantly full brain is expected, or decompression is needed, a larger craniotomy would be more favorable.[
LSO approach: General approach selection and technique considerations
The LSO craniotomy is employed primarily for anterior circulation lesions as a tailored adaptation of the established pterional craniotomy. It is a simpler version of the pterional approach and utilizes a smaller incision[
Figure 2:
Lateral supraorbital approach. (a) Supraorbital eyebrow, LSO, and pterional incisions. (b) Left LSO exposure. (c) Opticocarotid structures. (d) Oculomotor nerve followed back to the posterior circulation. Courtesy of the Rhoton Collection.[
Anterior circulation
With sufficient cerebrospinal fluid (CSF) release and brain relaxation, the LSO approach provides exceptional exposure to reach most anterior circulation lesions – with slight modifications based on specific lesions – to tailor the craniotomy more frontally or laterally. While most anterior circulation lesions may be reached comfortably through the LSO approach, those pathologies pointing posteriorly, such as a large anterior or PcomA aneurysm and giant MCA aneurysms, may prohibit one from using the LSO. In addition, limited exposure to lesions located in the distal anterior cerebral artery territory makes them a less suitable target for this approach.[
Posterior circulation
In experienced hands, high-riding basilar apex aneurysms may be reached with the LSO. The LSO allows access to the basilar tip, but it allows only a narrow corridor and a longer trajectory.[
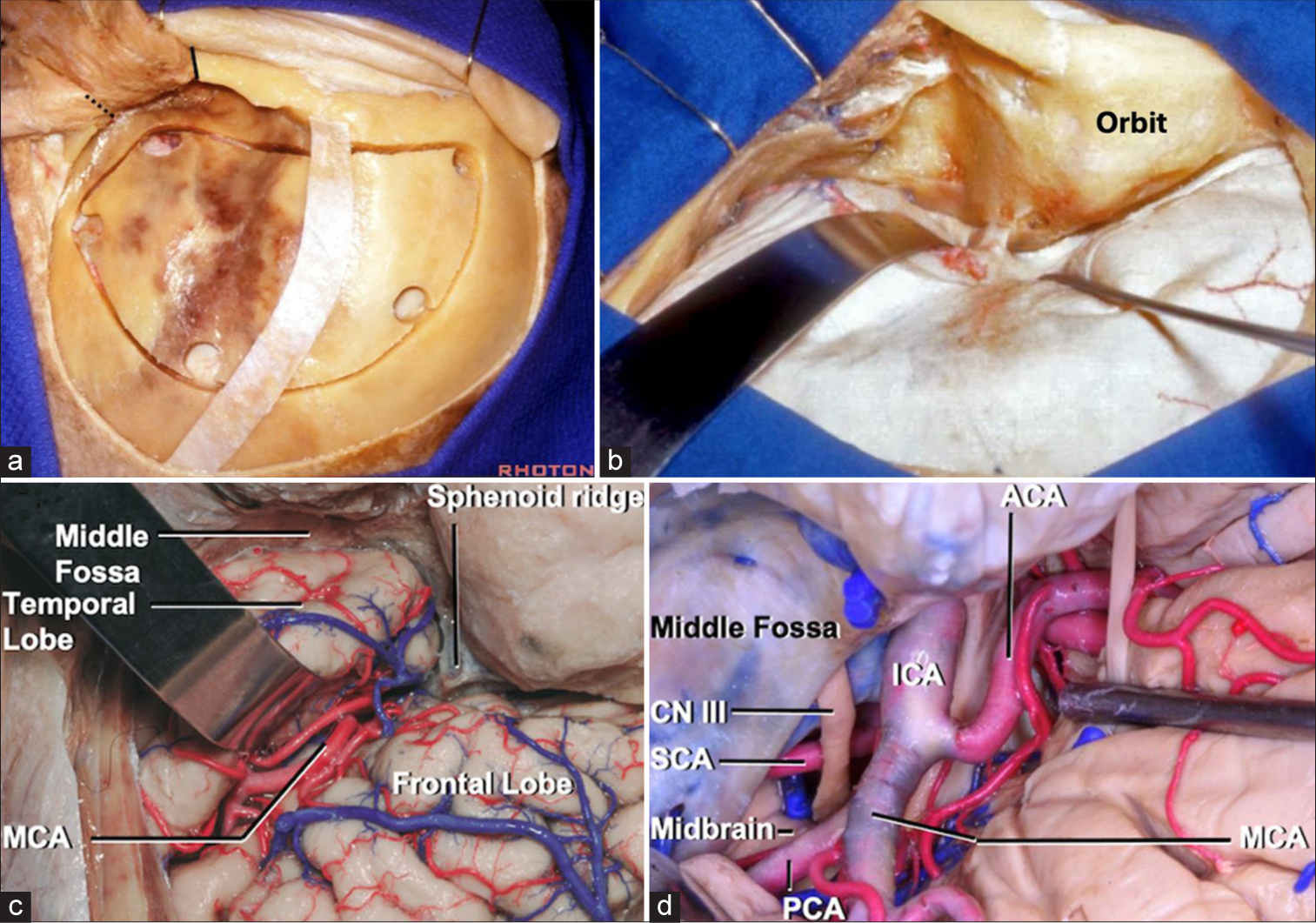
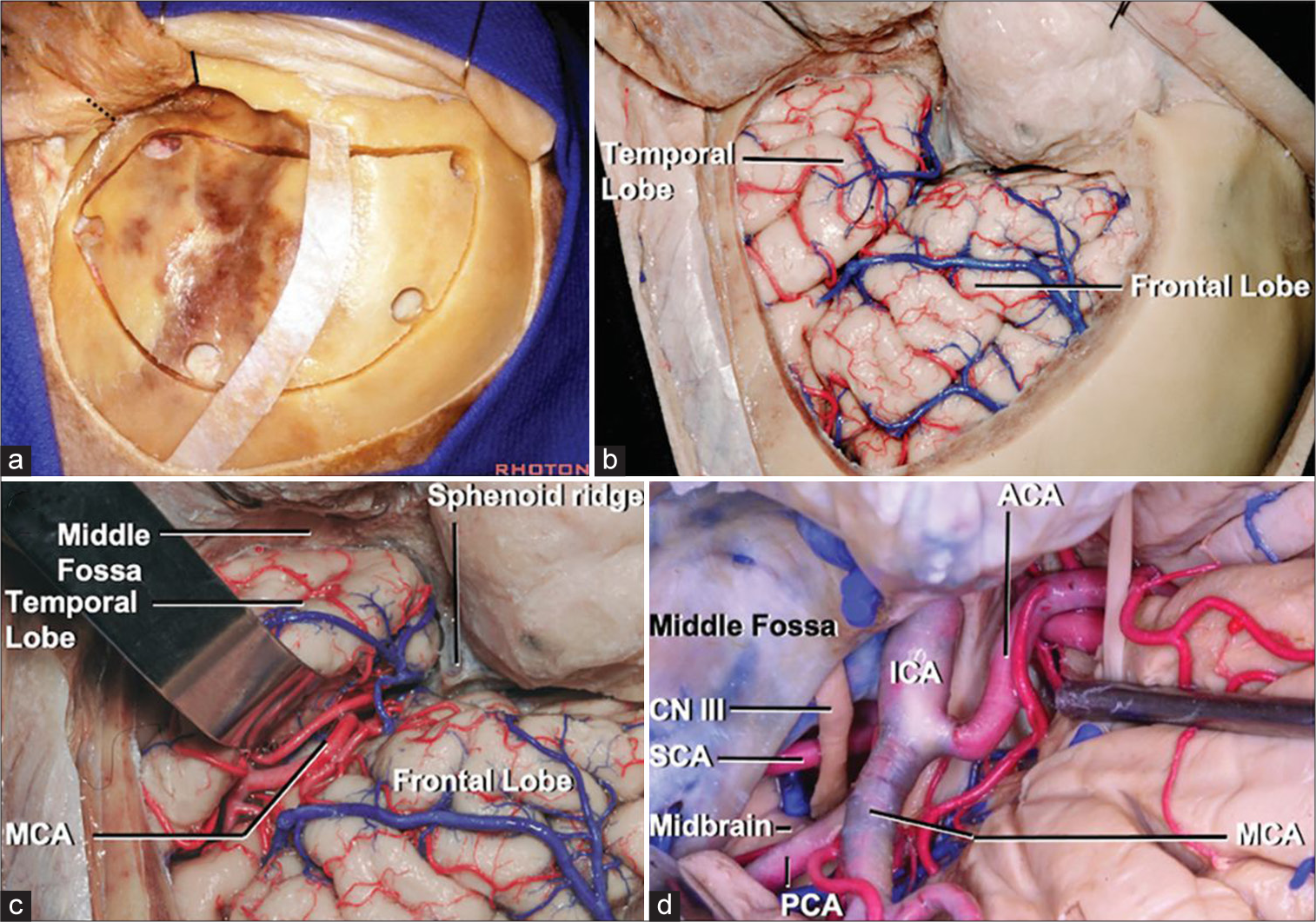
Cranio-orbital approach (“Inside-out”): General approach selection and technique considerations
Cranio-orbital craniotomies involve accessing the intraorbital compartment from within the cranial space, as opposed to the orbital-cranial approach, which accesses the intracranial compartment from the outside using the orbital corridor.[
Figure 3:
Cranio-orbital approach (“Inside-Out”). (a) Right modified one-piece craniotomy. McCarty keyhole accesses the frontal dura and periorbita. (b) Sylvian fissure exposure. (c) Exposure of anterior circulation. (d) Exposure of posterior circulation. Courtesy of the Rhoton Collection.[
Advantages and disadvantages of surgical targets
Anterior circulation
Exposure of anterior circulation lesions is maximized with a cranio-orbital approach. It allows direct access to the orbital apex, sphenoid ridge, and adjacent structures, making it suitable for a wide range of aneurysm locations in this region. It enables surgeons to attack from several angles, facilitating safe aneurysm clipping. It can be adapted and modified to access different types of aneurysms, including those in the anterior and posterior circulation.
Posterior circulation
To access superiorly situated lesions, such as high-riding basilar apex aneurysms or vascular malformations in this region, additional orbital removal may be required. This can be done as a separate two-piece osteotomy after performing a standard pterional craniotomy.[
In summary, the cranio-orbital approach offers excellent exposure and versatility for treating intracranial aneurysms, but it comes with potential cosmetic concerns, complexity, and risk of injury to orbital structures. Surgeons should carefully consider these factors when determining the most appropriate approach for each patient’s specific case.
Orbital-pterional approach (“Outside-in”): General approach selection and technique considerations
The cranio-orbital approach [
Figure 4:
Orbito-pterional approach (“Outside-In”). (a) Left pterional craniotomy. (b) Sylvian fissure. (c) Sylvian fissure dissection exposes the MCA. (d) The pretemporal approach, retracting the temporal pole backward, exposes the middle fossa dura, cavernous sinus, and posterior circulation. Courtesy of the Rhoton Collection.[
Advantages and disadvantages of approach for surgical targets
Anterior circulation
AcomA
The orbito-pterional craniotomy is an excellent working channel for accessing AcomA aneurysms. It obviates the need for splitting the Sylvian fissure and necessitates minimal brain retraction when accessing these aneurysms.[
Posterior circulation
The orbito-pterional approach permits access to the interpeduncular cistern, allowing access to the SCA and basilar apex aneurysms. Tayebi et al. found that the orbito-pterional approach offered improved PCA exposure and visualization of perforators, as well as an improved area of exposure to facilitate safe clipping of basilar apex aneurysms compared to a pterional approach.[
Trans-cavernous approach: General approach selection and technique considerations
The cavernous sinus is often considered a formidable operative target or approach pathway. Although endovascular treatment modalities are commonly employed for vascular pathology in this region, this approach is still considered a viable operative corridor, particularly to posterior circulation pathology not otherwise amenable to endovascular treatment.[
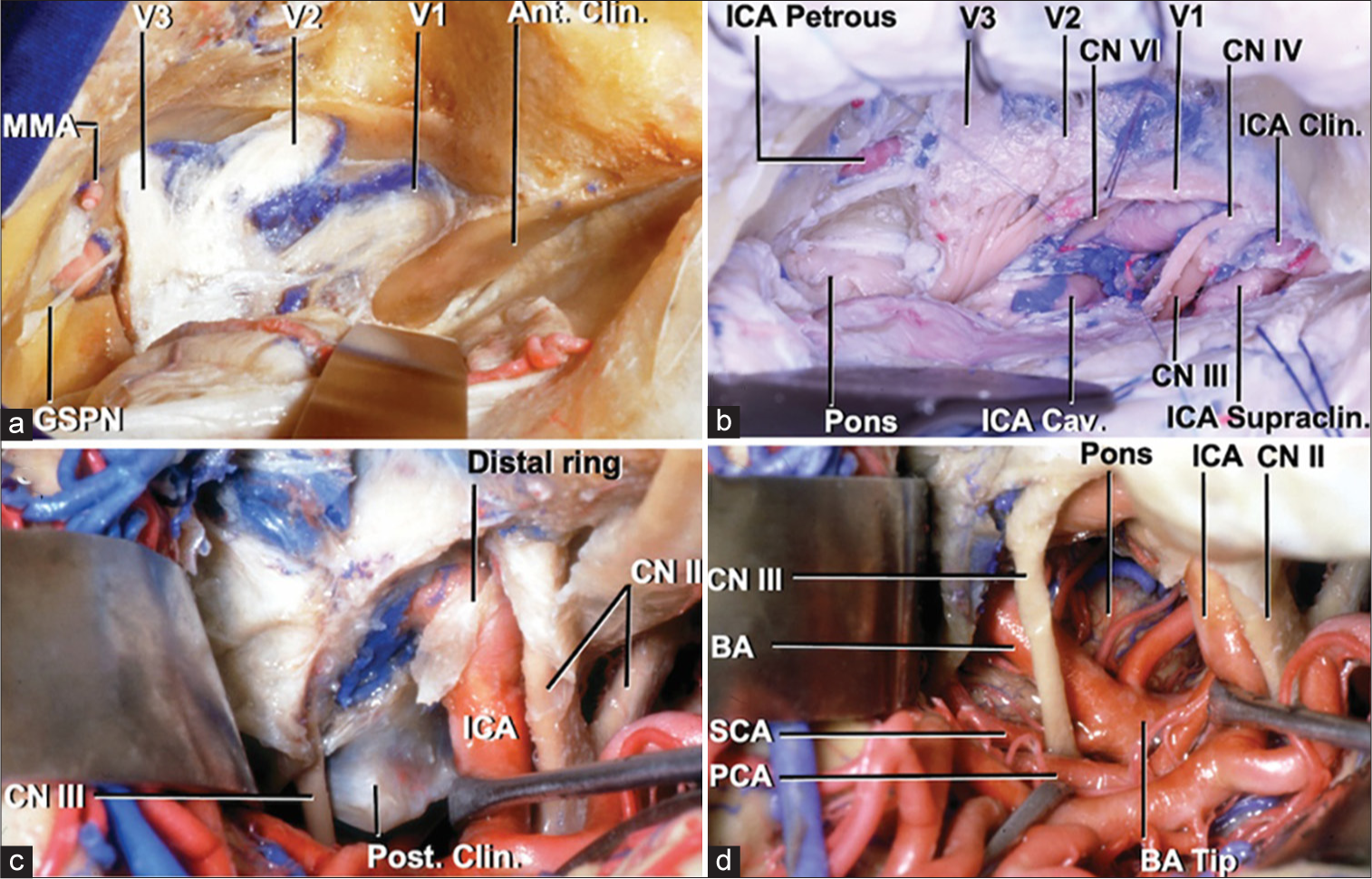
Figure 5:
Transcavernous approach. (a) Cavernous sinus exposure. (b) Exposure of cranial nerves, posterior fossa, ICA after anterior clinoidectomy and anterior petrosectomy. (c) Posterior clinoid between oculomotor nerve and ICA. (d) BA exposure after posterior clinoidectomy. Courtesy of the Rhoton Collection.[
Advantages and disadvantages of surgical targets
Anterior circulation
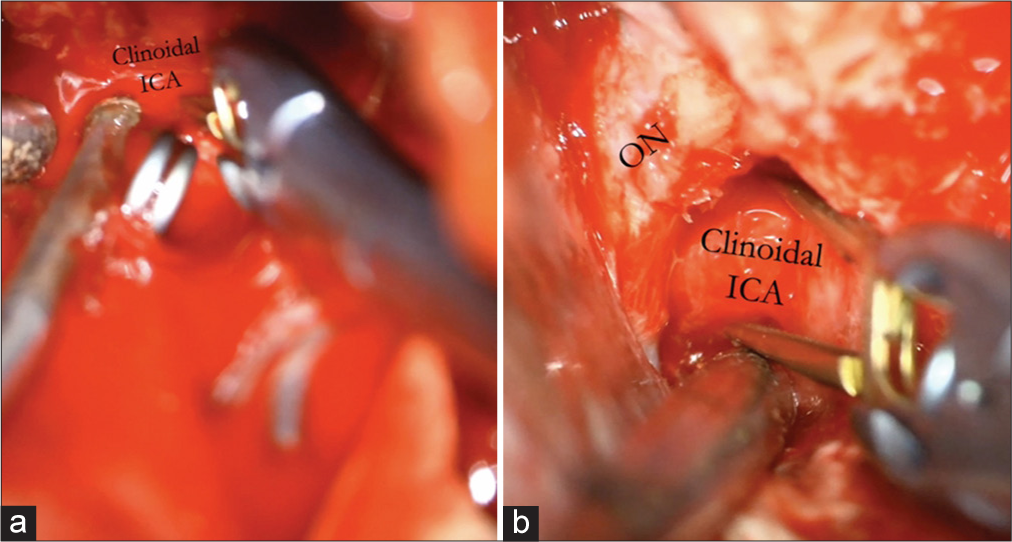
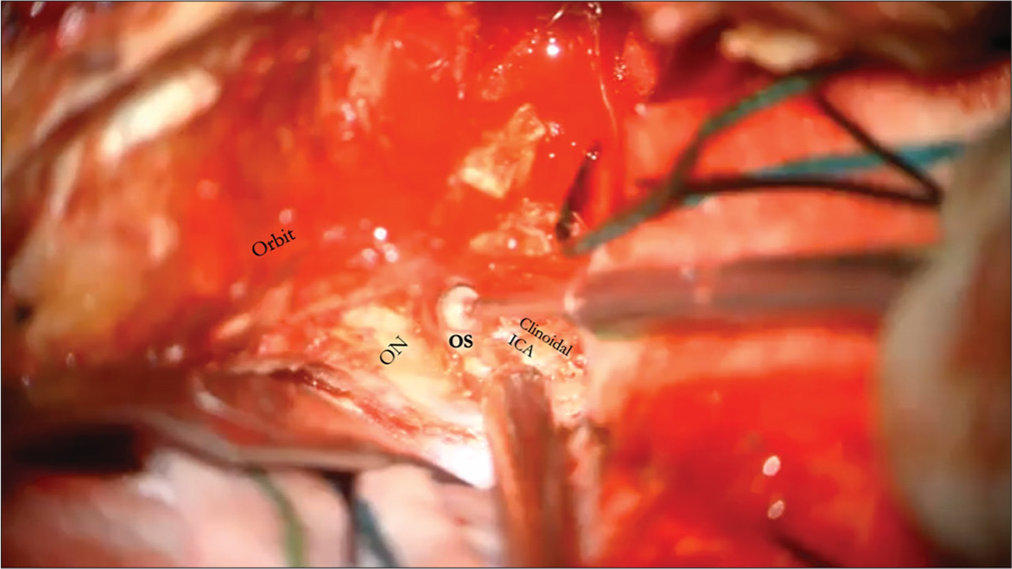
When accessing paraclinoidal aneurysms with this approach, incising the falciform ligament allows for the safer mobilization of the optic nerve. The dural ring is cut proximal to the takeoff of the ophthalmic artery to allow exposure to the aneurysm. At other times, the cavernous segment of the ICA must be accessed for proximal control. When this becomes necessary, such as when an aneurysm is abutting the clinoid process, access to the cavernous carotid can be accessed through Parkinson’s triangle.[
Posterior circulation
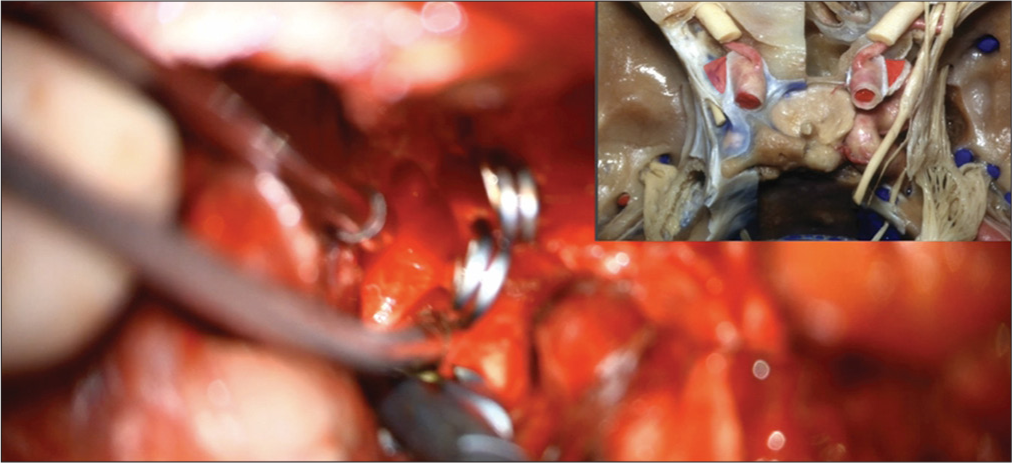
When a high-riding basilar apex aneurysm is being approached, access for proximal control requires mobilization of the oculomotor nerve, which is in the center of the operative field in this route to the basilar apex. It may also become necessary to divide the PcomA for unimpeded access to the interpeduncular fossa. In the case of low-lying basilar apex aneurysms, a circumferential view of the aneurysm for safe access and treatment can be achieved by performing a posterior clinoidectomy to expose the SCA and contralateral PCA to obtain proximal control.[
Case examples
Case 1
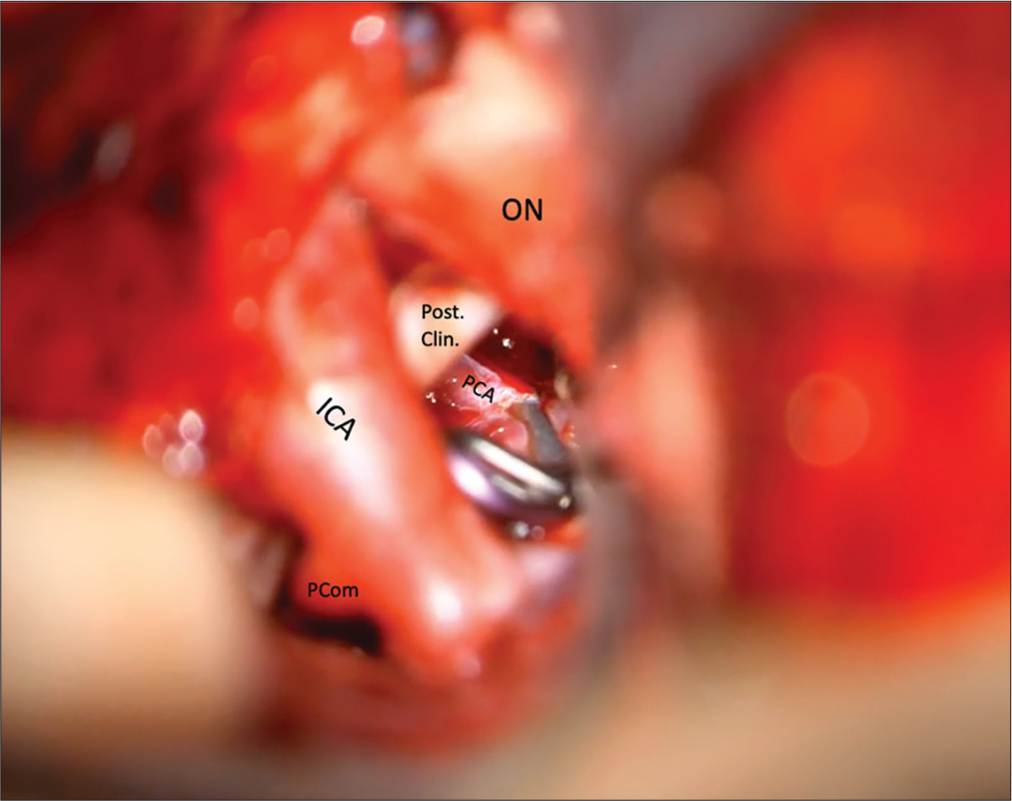
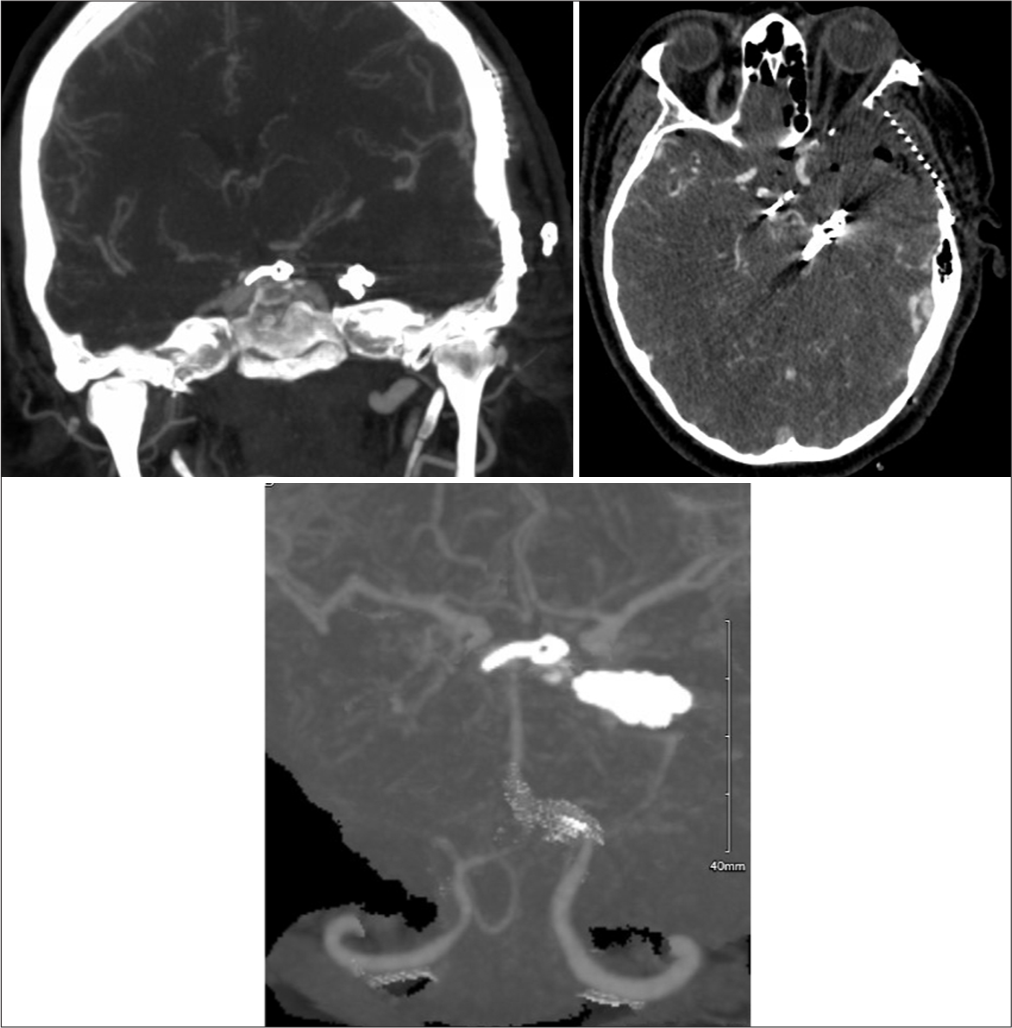
A 60-year-old female presented with a Hunt-Hess 2, Fisher 2 subarachnoid hemorrhage secondary to a ruptured PcomA aneurysm [
Case 2
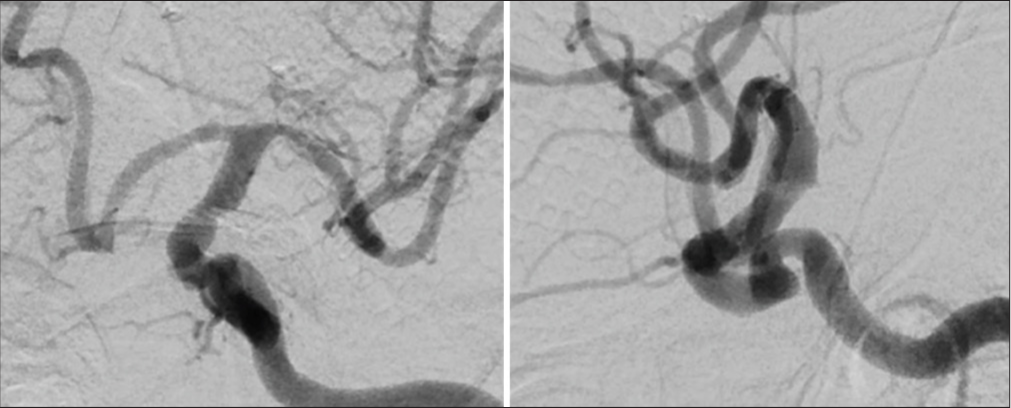
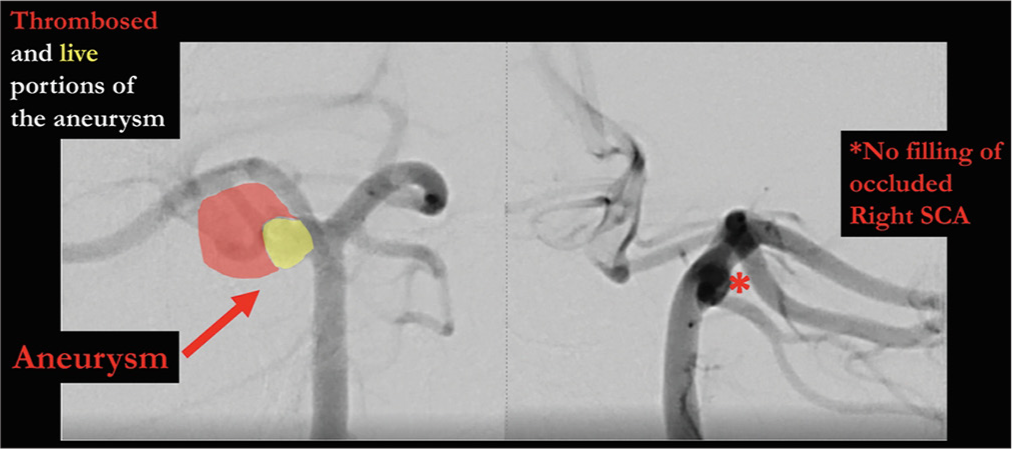
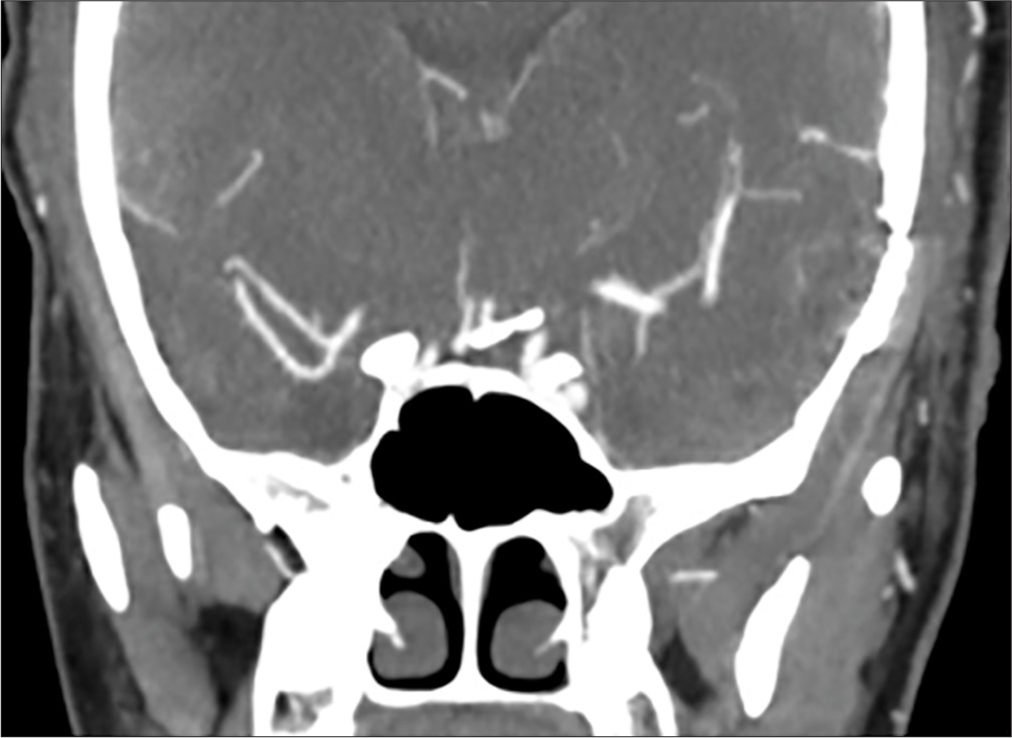
A 42-year-old male presented with acute severe headache, sudden left hemiplegia, and right oculomotor nerve palsy. Magnetic resonance imaging demonstrated a completed right SCA stroke. Computed tomography angiogram and formal angiography confirmed an unruptured large partially thrombosed right SCA aneurysm with mass effect occluding the right SCA, which was thought to be filling and growing [
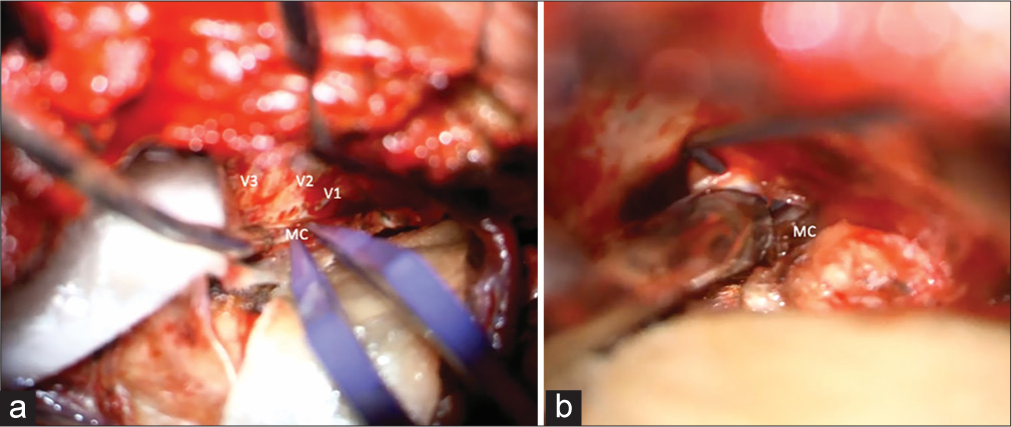
The patient underwent an orbito-pterional craniotomy, Hakuba “peeling” of the temporal dura propria from the lateral wall of the cavernous sinus, and anterior clinoidectomy [
Figure 18:
Postoperative CT angiogram with clip ligation of the aneurysm and bony work of partial anterior clinoidectomy (due to the middle clinoid process encircling the ICA) and posterior clinoidectomy can be visualized. CT: Computed tomography, ICA: Internal carotid artery. PCA: Posterior cerebral artery, SCA: Superior cerebellar artery.
Case 3
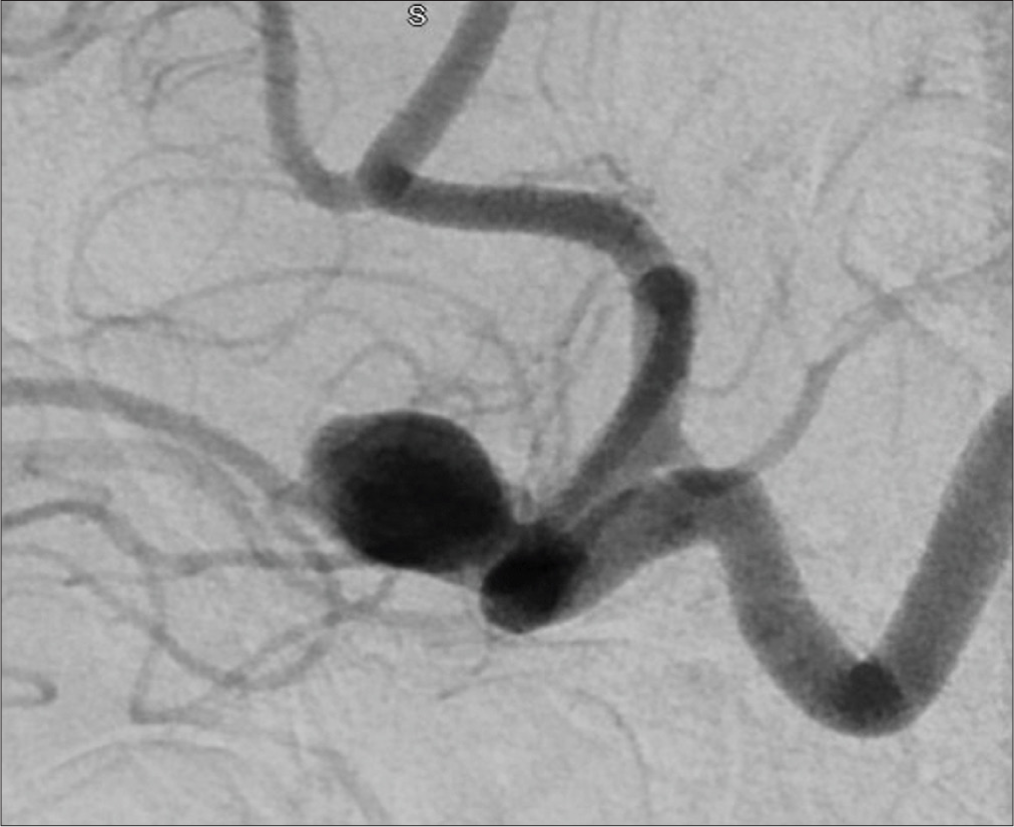
A 30-year-old female presented with a large unruptured left A1– A2 junction AcomA aneurysm with anterior inferior projection beneath and adherent to the left optic nerve. On examination, the patient is neurologically intact with no focal neurological deficits. The aneurysm was observed to have increased in size over a 2-month interval between initial and repeat angiography. Preoperative angiography is shown in
Initial attempts for endovascular treatment were made and aborted due to unfavorable anatomy. The patient underwent left LSO craniotomy with orbital roof osteotomy for clip ligation of the aneurysm [
Case 4
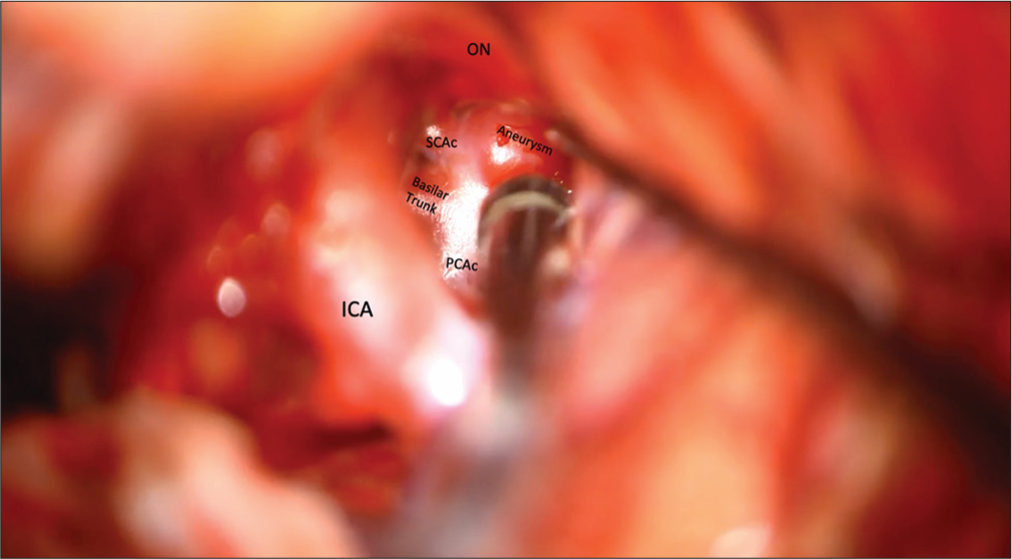
A 46-year-old female had previously presented to the hospital with headaches, incidental partially thrombosed left PCA fusiform aneurysm, and basilar apex aneurysm with no significant right P1 [
Figure 25:
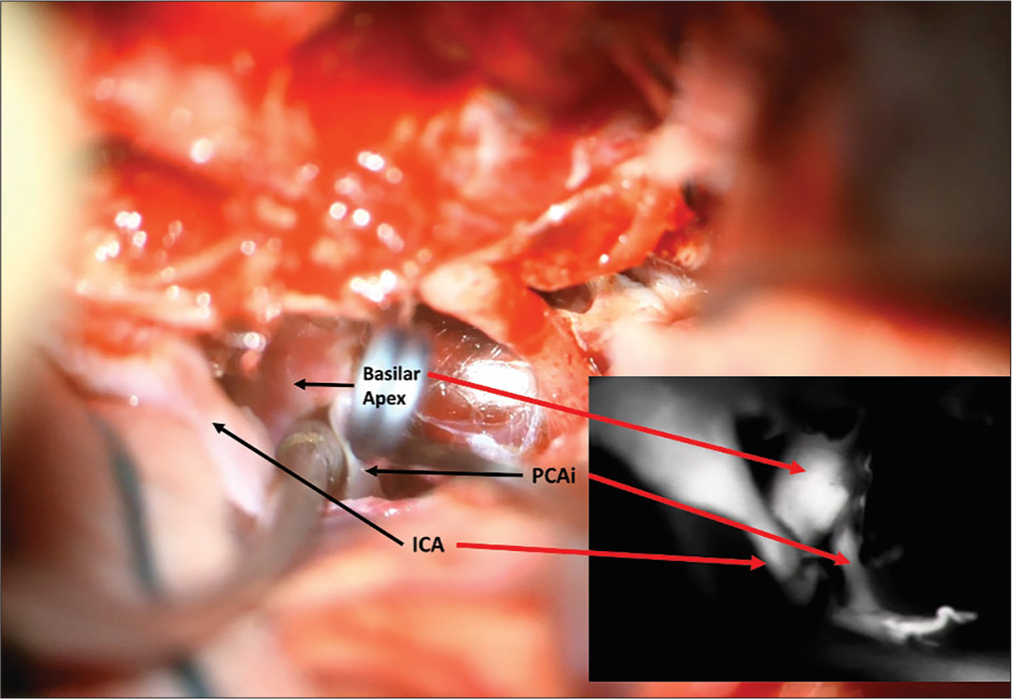
Aneurysm viewed through the transcavernous approach. Proximal control of the basilar artery was performed with temporary clipping utilizing the oculomotor triangle, but the angle of attack for clip ligation of the aneurysm was more optimal through the optic-carotid triangle. Lip ligation of the aneurysm was more optimal through the optic-carotid triangle.
CONCLUSION
Supraorbital, LSO, cranio-orbital (“outside-in”), and orbital-pterional (“inside-out”) craniotomies are approach modifications of the traditional pterional craniotomy. These different variations are useful for treating various vascular pathologies of the anterior and posterior circulation. The half-and-half or transcavernous approaches can be useful additions to these approaches. Understanding the advantages and disadvantages of each is important in optimal approach selection.
Ethical approval:
The Institutional Review Board approval is not required.
Declaration of patient consent:
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. AA, editors. The Rhoton collection. Boston, MA: American Association of Neurological Surgeons; 1975. p.
2. Abou-Al-Shaar H, Krisht KM, Cohen MA, Abunimer AM, Neil JA, Karsy M. Cranio-orbital and orbitocranial approaches to orbital and intracranial disease: Eye-opening approaches for neurosurgeons. Front Surg. 2020. 7: 1
3. Andaluz N, Van Loveren HR, Keller JT, Zuccarello M. Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery. 2003. 52: 1140-9
4. Andrade-Barazarte H, Kivelev J, Goehre F, Jahromi BR, Hijazy F, Moliz N. Contralateral approach to internal carotid artery ophthalmic segment aneurysms: Angiographic analysis and surgical results for 30 patients. Neurosurgery. 2015. 77: 104-12
5. Banerjee A, Brown B, Wadhwa R, Nourbakhsh A, Caldito G, Nanda A. When Is posterolateral orbitotomy useful in a pterional craniotomy? A morphometric study. Skull Base. 2011. 21: 147-52
6. Beretta F, Andaluz N, Chalaala C, Bernucci C, Salud L, Zuccarello M. Image-guided anatomical and morphometric study of supraorbital and transorbital minicraniotomies to the sellar and perisellar regions: Comparison with standard techniques: Laboratory investigation. J Neurosurg. 2010. 113: 975-81
7. Cha KC, Hong SC, Kim JS. Comparison between lateral supraorbital approach and pterional approach in the surgical treatment of unruptured intracranial aneurysms. J Korean Neurosurg Soc. 2012. 51: 334-7
8. Chalouhi N, Jabbour P, Ibrahim I, Starke RM, Younes P, El Hage G. Surgical treatment of ruptured anterior circulation aneurysms: Comparison of pterional and supraorbital keyhole approaches. Neurosurgery. 2013. 72: 437-42
9. Cikla U, Uluc K, Baskaya MK. Clip reconstruction of an 8 cm giant internal carotid artery bifurcation aneurysm: Microsurgical technique. Neurosurg Focus. 2015. 38: Video20
10. Da Silva SA, Yamaki VN, Solla DJ, Andrade AF, Teixeira MJ, Spetzler RF. Pterional, pretemporal, and orbitozygomatic approaches: Anatomic and comparative study. World Neurosurg. 2019. 121: e398-403
11. Dandy WE. Prechiasmal intracranial tumors of the optic nerves. Am J Ophthalmol. 1922. 5: 169-88
12. Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983. 58: 824-31
13. Esposito G, Dias SF, Burkhardt JK, Fierstra J, Serra C, Bozinov O. Selection strategy for optimal keyhole approaches for middle cerebral artery aneurysms: Lateral supraorbital versus minipterional craniotomy. World Neurosurg. 2019. 122: e349-57
14. Figueiredo EG, Zabramski JM, Deshmukh P, Crawford NR, Spetzler RF, Preul MC. Comparative analysis of anterior petrosectomy and transcavernous approaches to retrosellar and upper clival basilar artery aneurysms. Oper Neurosurg. 2006. 58: ONS13-21
15. Hakuba A, Nishimura S, Shirakata S, Tsukamoto M. Surgical approaches to the cavernous sinus: Report of 19 cases. Neurol Med Chir (Tokyo). 1982. 22: 295-308
16. Hernesniemi J, Andrade-Barazarte H, Duarte R, Serrone J, Hijazy F, July J, Wahjoepramono EJ, editors. Lateral supraorbital approach. Neurovascular surgery. Singapore: Springer; 2019. p. 23-8
17. Karadimas S, Shakil H, Almeida JP, Tymianski M, Radovanovic I. Minimally invasive and outpatient aneurysm surgery. Neurosurg Clin N Am. 2022. 33: 371-82
18. Lan Q, Zhang H, Zhu Q, Chen A, Chen Y, Xu L. Keyhole approach for clipping intracranial aneurysm: Comparison of supraorbital and pterional keyhole approach. World Neurosurg. 2017. 102: 350-9
19. Najera E, Muhsen BA, Borghei-Razavi H, Obrzut M, Adada B. Transcavernous approach for microsurgical clipping of ruptured right superior cerebellar artery aneurysm with cadaveric stepwise video illustration. J Neurol Surg Part B Skull Base. 2022. 83: e619-20
20. Nguyen V, Basma J, Klimo P, Sorenson J, Michael L. Orbitopterional approach for the resection of a suprasellar craniopharyngioma: Adapting the strategy to the microsurgical and pathologic anatomy. J Neurol Surg Part B Skull Base. 2018. 79: S239-40
21. Ormond DR, Hadjipanayis CG. The Supraorbital keyhole craniotomy through an eyebrow incision: Its origins and evolution. Minim Invasive Surg. 2013. 2013: 296469
22. Park J. Supraorbital keyhole approach for intracranial aneurysms: Transitioning from concerns to confidence. J Korean Neurosurg Soc. 2020. 63: 4-13
23. Rai HI, Kayssi AR, Krisht A. Pretemporal transcavernous approach to basilar tip aneurysms: Operative technique and surgical nuances: 2-dimensional operative video. Oper Neurosurg. 2023. 26: 226-7
24. Salma A, Alkandari A, Sammet S, Ammirati M. Lateral supraorbital approach vs pterional approach: An anatomic qualitative and quantitative evaluation. Oper Neurosurg. 2011. 68: ons364-72
25. Schwartz MS, Anderson GJ, Horgan MA, Kellogg JX, McMenomey SO, Delashaw JB. Quantification of increased exposure resulting from orbital rim and orbitozygomatic osteotomy via the frontotemporal transsylvian approach. J Neurosurg. 1999. 91: 1020-6
26. Seoane E, Tedeschi H, De Oliveira E, Wen HT, Rhoton AL. The Pretemporal transcavernous approach to the interpeduncular and prepontine cisterns: Microsurgical anatomy and technique application. Neurosurgery. 2000. 46: 891-9
27. Snyderman CH, Gardner PA, editors. Master techniques in otolaryngology - head and neck surgery: Skull base surgery. Philadelphia, PA: Wolters Kluwer; 2015. p.
28. Tanriover N, Ulm AJ, Rhoton AL, Kawashima M, Yoshioka N, Lewis SB. One-piece versus two-piece orbitozygomatic craniotomy: Quantitative and qualitative considerations. Oper Neurosurg. 2006. 58: ONS229-37
29. Tayebi Meybodi A, Benet A, Rodriguez Rubio R, Yousef S, Lawton MT. Comprehensive anatomic assessment of the pterional, orbitopterional, and orbitozygomatic approaches for basilar apex aneurysm clipping. Oper Neurosurg. 2018. 15: 538-50