- Research Center for Neuromodulation and Pain, Shiraz University of Medical Sciences, Shiraz, Iran,
- Division of Neurosurgery, City of Hope Beckman Research Institute and Medical Center, Duarte, California, United States.
Correspondence Address:
Ali Razmkon, Research Center for Neuromodulation and Pain, Shiraz University of Medical Sciences, Shiraz, Iran.
DOI:10.25259/SNI_435_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Omid Yousefi1, Mojtaba Dayyani2, Razieh Rezaei1, Hooman Kamran1, Ali Razmkon1. Deep brain stimulation of the posterior subthalamic area as an alternative strategy for management of Holmes tremor: A case report and review of the literature. 28-Oct-2022;13:489
How to cite this URL: Omid Yousefi1, Mojtaba Dayyani2, Razieh Rezaei1, Hooman Kamran1, Ali Razmkon1. Deep brain stimulation of the posterior subthalamic area as an alternative strategy for management of Holmes tremor: A case report and review of the literature. 28-Oct-2022;13:489. Available from: https://surgicalneurologyint.com/surgicalint-articles/deep-brain-stimulation-of-the-posterior-subthalamic-area-as-an-alternative-strategy-for-management-of-holmes-tremor-a-case-report-and-review-of-the-literature/
Abstract
Background: Holmes tremor is often refractory to medical treatment and deep brain stimulation of the ventralis intermedius nucleus of the thalamus (VIM-DBS) is the intervention of choice in controlling the tremor. Herein, we present a beneficial alternative strategy for the management of such situations, considering the posterior subthalamic area (PSA) as the target of stimulation.
Case Description: We report a 57-year-old male with the right-sided tremor following a traumatic brain injury 20 years ago. He had been diagnosed with Holmes tremor that was not responsive to nonsurgical therapeutic options. When refractoriness confirmed, he became a candidate for VIM-DBS. During the operation, by performing macrostimulation with a maximum of 2 mA of amplitude, the tremor had no response to the stimulation of different tracts, and severe right hemi-body paresthesia occurred; therefore, we modified our approach and targeted the PSA, which resulted in satisfactory control of the tremor. The permanent lead was implanted into the left side PSA. At 1-year follow-up, the right side tremor was under complete control.
Conclusion: Our case and other similar pieces of evidence are consistently indicating the potential regulatory effects of PSA-DBS in controlling the Holmes tremor as a feasible alternative strategy when VIM-DBS does not provide a satisfactory response. However, further studies with larger sample size are required to evaluate the long-term response and its possible long-term stimulation-related effects.
Keywords: Deep brain stimulation, Holmes tremor, Nucleus ventralis intermedius, Posterior subthalamic area, Thalamus, Tremor
INTRODUCTION
High-frequency stimulation and lesioning of the nucleus ventralis intermedius (VIM) are the conventional surgical procedures used for alleviating tremors of different pathologies. VIM-deep brain stimulation (DBS) was first introduced as the surgical treatment for Parkinson’s disease, and its safety and efficacy for the treatment of tremor dominant movement disorders such as essential tremor (ET) and Holmes tremor (HT) have been reported in several studies.[
HT, caused by a lesion in the basal ganglia and specific tracts, is believed to have a low response to medical treatments.[
The posterior subthalamic area (PSA) is among the targets and efficient in controlling the tremor by receiving high-frequency stimulation.[
We present a 57-year-old male with HT who had no response to medical treatment for 20 years and eventually became a candidate for VIM-DBS. Due to the weak response to VIM-DBS during surgery, the PSA-DBS was chosen as the alternative strategy. Herein, we discuss the rationale, technical challenges, and outcome of the PSA-DBS strategy, along with a comprehensive review of the pertinent literature.
CASE REPORT
A 57-year-old otherwise healthy male had fallen off a horse 20 years ago. He had had a traumatic brain injury and had been in coma for a month. Immediately after gaining consciousness, he developed dysarthria, and 6 months after the accident, he gradually developed right-sided hemiparesis and hemi-body tremor. Resting, action, and positional tremor have been significantly debilitating the patient and hindering him to perform his daily activities. He had also been unable to walk even with assistance and had been wheelchair bounded for the past 20 years.
The patient had received various medical treatments over the years, and none of them was effective in the control of the tremor. Eventually, he was referred to our movement disorder clinic in February 2020. As the possible therapeutic surgical intervention to tackle his tremor, unilateral VIMDBS was suggested to him and his family.
Surgical procedure
Nonstereotactic magnetic resonance imaging (MRI) (1.5 T Integra, Philips, Netherlands) was obtained a few days before surgery under light sedation (to prevent motion artifacts secondary to tremor). On the day of surgery, after installing the Cosman–Roberts–Wells (CRW) stereotactic frame (Integra Life, USA), a stereotactic computed tomography (CT) scan was done.
The planning process was carried out using StealthStation S8 (Medtronic, Minneapolis, USA). Initially, gadolinium-enhanced T1-weighted MRI was selected as a reference, and other MRI sequences and CT scan were superimposed on it and checked if the different sets of landmarks were matched accurately. After defining the anterior and posterior commissures on the axial T1 sequences, indirect targeting of VIM was accomplished through axial and coronal T2-weighted sequences. The stereotactic coordinates of the VIM target were X: −14.6, Y: −5, and Z: −2.
Following the localization of the entrance point and the trajectory, microelectrode recording (MER) was performed using the Leadpoint system (Medtronic, Skovlundae, Denmark, and Shoreview, Minnesota, USA). The action potentials were recorded in the medial, central, and posterior tracts. The recording suggestive of the thalamic tremor was captured in all tracts from 4 mm above to 2 mm below the level of the target. Based on these findings, we decided to perform macrostimulation along the central tract. The high-frequency constant current stimulation was initiated from 4 mm above the target, under the supervision of a movement disorder neurologist. While stimulation was started from 0 mA and was increased stepwise by 1 mA at each level, the patients’ tremor was getting assessed continuously along with the ascending stimulation. The gradual increment of stimulation was cautiously continued till any stimulation-related adverse event was observed. Unfortunately, the stimulation was not effective in the central, posterior, medial, and anterior tracts. Resting, action, and intentional tremors did not respond to the stimulation with different amplitudes, and when the amplitude reached 2 mA, severe acute right hemi-body paresthesia was evident as an adverse event of stimulation. Using intraoperative lateral fluoroscopy and trunion reticles, we confirmed that the stimulation electrode was precisely placed in the correct location.
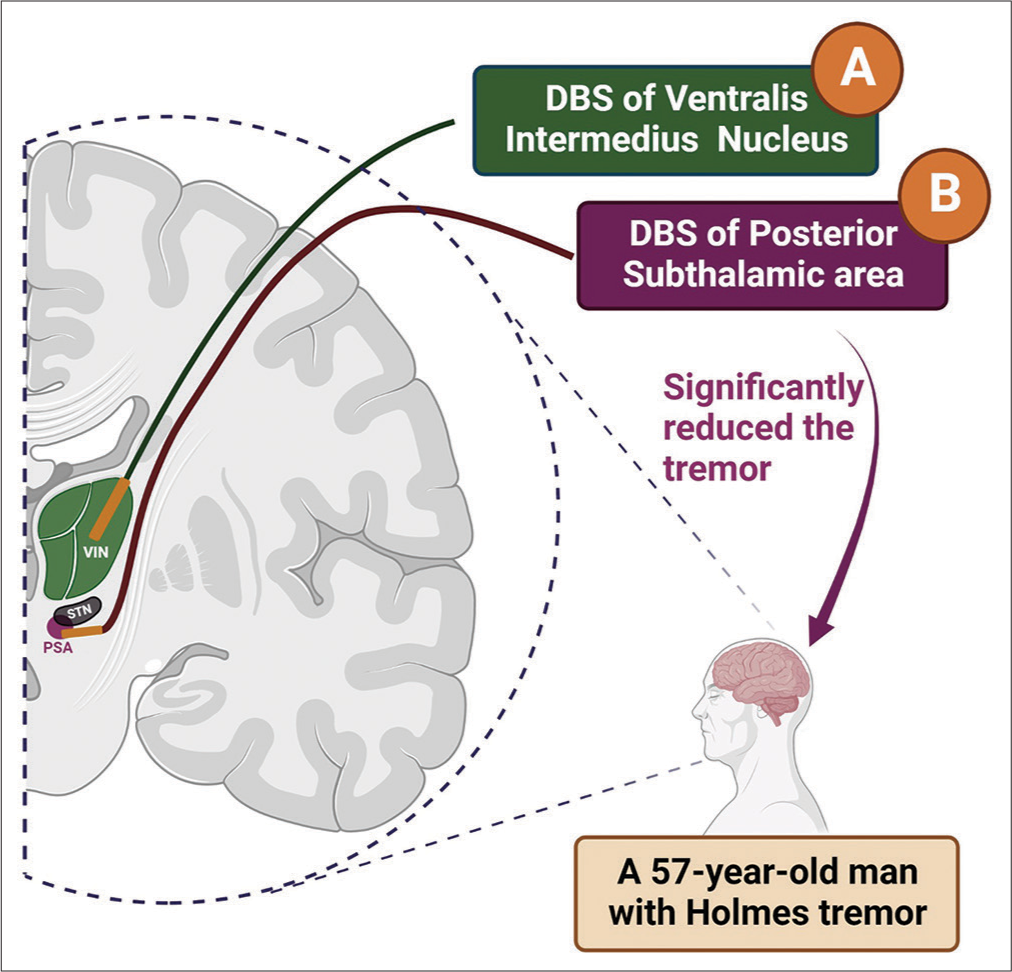
Nonetheless, due to the insufficient response to VIM stimulation, we decided to perform macrostimulation of PSA as a possible alternative strategy [
Figure 1:
Conventional VIM-DBS versus the alternative approach, PSA-DBS.
A: Deep brain stimulation of VIM as the conventional intervention of choice was not helpful in reducing tremor. B: Deep brain stimulation of PSA as the alternative strategy resulted in satisfactory control of the tremor. #Trajectories of the leads demonstrated in this figure are putative and do not represent the actual tracts in the surgery. (The figure is created with BioRender.com).
Interestingly, PSA stimulation (2 mA) resulted in a considerable alleviation of all types of tremors. The patient and his family were reinformed about the favorable response of PSA-DBS, and after obtaining the consent, the permanent lead (DB-2201, Boston Scientific, USA) was implanted in the left PSA in a way that contact 1 was placed 2 mm below the defined target.
An immediate postoperative CT scan was done and the images were superimposed on the preoperative imaging to check the final location of the lead and any possible surgical inaccuracy or complications. The CT scan showed no misplacement or deviation of the lead from the defined target. As the last step, the neurostimulator (Vercise PC, Boston Scientific, USA) was implanted in the right upper part of the chest under general anesthesia. We avoided the left side insertion because implanted pulse generator (IPG) would interfere with the function of implanted cardiac devices (ICD) if the patient ever needed an ICD. After recovery, the patient was transferred to the intensive care unit and then transferred to the ward after 1 day.
Follow-up
The initial programming was performed 2 weeks after the surgery. Contact No. 2 of the implanted lead was selected as the active contact, and the unipolar stimulation parameters were adjusted as follows: Amplitude: 1.5 mA, pulse width: 60 µs, and frequency: 130 Hz.
Afterward, the tremor was significantly controlled and the patient gradually gained the ability to have a walker-assisted gait within 10 months and could accomplish his daily activities. At the time of the 1-year follow-up, he had an infrequent mild tremor and needed modest assistance with gait. Furthermore, no stimulation-related adverse event was observed, and the patient and his family were completely satisfied with the outcome of the surgery. Due to the unprogressive and completely alleviated tremor at the 1-year follow-up, the stimulation parameters were not readjusted at that point.
DISCUSSION
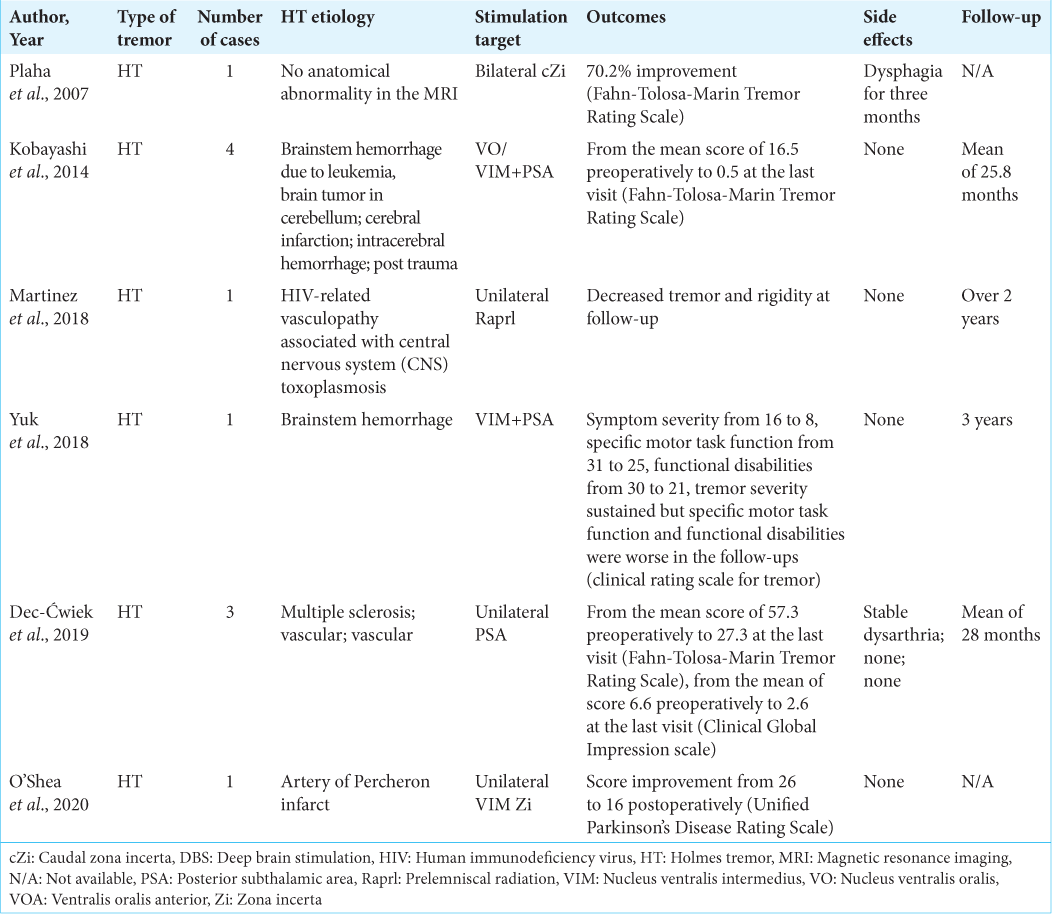
Among the movement disorders, HT usually does not show a satisfactory response to medical treatment. There are pieces of evidence indicating the efficacy of the surgical intervention, including DBS or lesioning, for the management of this disorder.[
VIM has been the conventional target used for the surgical treatment of tremor; however, new targets have been applied for cases in whom stimulation of the VIM seemed to be ineffective.[
Before the routine application of DBS, lesioning of PSA was a common procedure for the surgical management of tremor and Parkinson’s disease.[
In another study by Blomstedt et al.,[
There are a few reports in the literature representing the application of PSA-DBS on HT.
Furthermore, some studies suggest dual-target stimulation [
Some studies have done head to head comparison between the effectiveness of PSA-DBS and VIM-DBS. In a randomized and clinical trial by Barbe et al.,[
As the cerebello-thalamo-cortical circuit is related to ET, interfering with this circuit may alleviate the tremor.[
The exact underlying mechanism of tremor in HT is not known, which explains why the effectiveness of PSA-DBS has not been sharply demonstrated as well.[
Despite the advantages, PSA-DBS can be accompanied by stimulation-related adverse events. A study by Kim et al.[
Furthermore, a mini-review assessing the efficacy of PSADBS in patients with various types of tremors, including multiple sclerosis and posttraumatic-induced tremor, cerebellar tremor, HT, and spinocerebellar ataxia, reported that complications including dysphasia, dysarthria, or disequilibrium were mostly mild, transient, and not accompanied by tolerance, while the latter can be observed among the subjects who undergo VIM-DBS.[
CONCLUSION
In HT cases that are unresponsive to conventional VIMDBS, PSA-DBS can be a potential substitution approach to control the tremor effectively. However, almost all supporting evidence are limited to a few case reports. Therefore, further studies, especially the ones with larger sample size and possibly quasi-controlled designs, are needed to justify its potential efficacy along with the limitations and challenges compared to other targets, especially VIM.
Statement of ethics
The Ethics Committee of the Research Center for Neuromodulation and Pain (Shiraz, Iran) approved this study, and written informed consent from the presented case was obtained.
Data availability statement
All relevant data have been reported in the manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We are grateful to our cooperative operating room staff and our patient and his family for trusting us to accomplish this uncommon surgical strategy.
References
1. Al-Fatly B, Ewert S, Kübler D, Kroneberg D, Horn A, Kühn AA. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain. 2019. 142: 3086-98
2. Aydin S, Abuzayed B, Kiziltan G, Gunduz A, Yagci S, Mengi M. Unilateral thalamic vim and GPi stimulation for the treatment of Holmes’ tremor caused by midbrain cavernoma: Case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2013. 74: 271-6
3. Aydin S, Canaz H, Erdogan ET, Durmaz N, Topcular B. Holmes’ tremor with shoulder pain treated by deep brain stimulation of unilateral ventral intermediate thalamic nucleus and globus pallidus internus. J Mov Disord. 2017. 10: 92-5
4. Bandt SK, Anderson D, Biller J. Deep brain stimulation as an effective treatment option for post-midbrain infarction-related tremor as it presents with benedikt syndrome. J Neurosurg. 2008. 109: 635-9
5. Barbe MT, Reker P, Hamacher S, Franklin J, Kraus D, Dembek TA. DBS of the PSA and the VIM in essential tremor. Neurology. 2018. 91: e543-50
6. Blomstedt P, Fytagoridis A, Åström M, Linder J, Forsgren L, Hariz MI. Unilateral caudal zona incerta deep brain stimulation for Parkinsonian tremor. Parkinsonism Relat Disord. 2012. 18: 1062-6
7. Blomstedt P, Fytagoridis A, Tisch S. Deep brain stimulation of the posterior subthalamic area in the treatment of tremor. Acta Neurochir (Wien). 2009. 151: 31-6
8. Blomstedt P, Sandvik U, Fytagoridis A, Tisch S. The posterior subthalamic area in the treatment of movement disorders: Past, present, and future. Neurosurgery. 2009. 64: 1029-38 discussion 1038-42
9. Blomstedt P, Sandvik U, Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord. 2010. 25: 1350-6
10. Blomstedt P, Persson RS, Hariz GM, Linder J, Fredricks A, Häggström B. Deep brain stimulation in the caudal zona incerta versus best medical treatment in patients with Parkinson’s disease: A randomised blinded evaluation. J Neurol Neurosurg Psychiatry. 2018. 89: 710-6
11. Coenen VA, Allert N, Paus S, Kronenburger M, Urbach H, Madler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: A diffusion tensor imaging study. Neurosurgery. 2014. 75: 657-9 discussion 669-70
12. Dec-Ćwiek M, Tutaj M, Pietraszko W, Libionka W, Krupa M, Moskała M. Posterior subthalamic area deep brain stimulation for treatment of refractory Holmes tremor. Stereotact Funct Neurosurg. 2019. 97: 183-8
13. Dembek TA, Petry-Schmelzer JN, Reker P, Wirths J, Hamacher S, Steffen J. PSA and VIM DBS efficiency in essential tremor depends on distance to the dentatorubrothalamic tract. Neuroimage Clin. 2020. 26: 102235
14. Fan H, Bai Y, Yin Z, An Q, Xu Y, Gao Y. Which one is the superior target? A comparison and pooled analysis between posterior subthalamic area and ventral intermediate nucleus deep brain stimulation for essential tremor. CNS Neurosci Ther. 2022. 28: 1380-92
15. Foote KD, Okun MS. Ventralis intermedius plus ventralis oralis anterior and posterior deep brain stimulation for posttraumatic Holmes tremor: Two leads may be better than one: Technical note. Neurosurgery. 2005. 56: E445 discussion E445
16. Foote KD, Seignourel P, Fernandez HH, Romrell J, Whidden E, Jacobson C. Dual electrode thalamic deep brain stimulation for the treatment of posttraumatic and multiple sclerosis tremor. Neurosurgery. 2006. 58: ONS-280-5 discussion ONS-285-6
17. Fytagoridis A, Blomstedt P. Complications and side effects of deep brain stimulation in the posterior subthalamic area. Stereotact Funct Neurosurg. 2010. 88: 88-93
18. Houdart R, Mamo H, Dondey M, Cophignon J. Results of subthalamic coagulations in Parkinson’s disease (apropos of 50 cases). Rev Neurol (Paris). 1965. 112: 521-9
19. Kim MC, Son BC, Miyagi Y, Kang JK. Vim thalamotomy for Holmes’s tremor secondary to midbrain tumour. J Neurol Neurosurg Psychiatry. 2002. 73: 453-5
20. Kim MJ, Chang KW, Park SH, Chang WS, Jung HH, Chang JW. Stimulation-induced side effects of deep brain stimulation in the ventralis intermedius and posterior subthalamic area for essential tremor. Front Neurol. 2021. 12: 678592
21. Kobayashi K, Katayama Y, Oshima H, Watanabe M, Sumi K, Obuchi T. Multitarget, dual-electrode deep brain stimulation of the thalamus and subthalamic area for treatment of Holmes’ tremor. J Neurosurg. 2014. 120: 1025-32
22. Koller WC, Pahwa PR, Lyons KE, Wilkinson SB. Deep brain stimulation of the vim nucleus of the thalamus for the treatment of tremor. Neurology. 2000. 55: S29-33
23. Martinez V, Hu SC, Foutz TJ, Ko A. Successful treatment of Holmes tremor with deep brain stimulation of the prelemniscal radiations. Front Surg. 2018. 5: 21
24. Merchant SH, Kuo SH, Qiping Y, Winfield L, McKhann G, Sheth S. Objective predictors of ‘early tolerance’ to ventral intermediate nucleus of thalamus deep brain stimulation in essential tremor patients. Clin Neurophysiol. 2018. 129: 1628-33
25. Mundinger F. New stereotactic treatment of spasmodic torticollis with a brain stimulation system (author’s transl). Med Klin. 1977. 72: 1982-6
26. O’Shea SA, Elkind M, Pullman SL, Ford B. Holmes tremor due to artery of percheron infarct: Clinical case and treatment using deep brain stimulation of the vim and ZI targets. Tremor Other Hyperkinet Mov (N Y). 2020. 10: 732
27. Oliveria SF, Rodriguez RL, Bowers D, Kantor D, Hilliard JD, Monari EH. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: A single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 2017. 16: 691-700
28. Papuć E, Obszańska K, Trojanowski T, Szczepańska-Szerej H, Rejdak K, Stelmasiak Z. Reduction of thalamic tremor with deep brain stimulation performed for post stroke chronic central pain. Ann Agric Environ Med. 2013. 20: 45-7
29. Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008. 79: 504-13
30. Ramirez-Zamora A, Okun MS. Deep brain stimulation for the treatment of uncommon tremor syndromes. Expert Rev Neurother. 2016. 16: 983-97
31. Romanelli P, Brontë-Stewart H, Courtney T, Heit G. Possible necessity for deep brain stimulation of both the ventralis intermedius and subthalamic nuclei to resolve Holmes tremor. Case report. J Neurosurg. 2003. 99: 566-71
32. Schneider SA, Deuschl G. Medical and surgical treatment of tremors. Neurol Clin. 2015. 33: 57-75
33. Story JL, French LA, Chou SN, Meier MJ. Experiences with subthalamic lesions in patients with movement disorders. Confin Neurol. 1965. 26: 218-21
34. Thompson AJ, Peng-Chen Z, Pastrana M, Foote KD, Haq I, Okun MS. Intraoperative smile in a multiple sclerosis patient with medication-refractory tremor. Neurocase. 2014. 20: 698-703
35. Toda H, Nishida N, Iwasaki K. Coaxial interleaved stimulation of the thalamus and subthalamus for treatment of Holmes tremor. Neurosurg Focus. 2017. 42: V1
36. Wang KL, Wong JK, Eisinger RS, Carbunaru S, Smith C, Hu W. Therapeutic advances in the treatment of Holmes tremor: Systematic review. Neuromodulation. 2022. 25: 796-803
37. Xie T, Bernard J, Warnke P. Post subthalamic area deep brain stimulation for tremors: A mini-review. Transl Neurodegener. 2012. 1: 20
38. Yuk CD, Ahn JH, Oh JK, Chang IB, Song JH, Kim JH. Deep brain stimulation of the ventralis intermedius nucleus of the thalamus and posterior subthalamic area for Holmes’ tremor secondary to brainstem hemorrhage: A case report. J Clin Neurosci. 2019. 60: 160-4