- Department of Neurosurgery, Allegheny Health Network, Pittsburgh, United States
- Department of Hematology/Oncology, Allegheny Health Network, Pittsburgh, United States
Correspondence Address:
Trent Kite, Department of Neurosurgery, Allegheny Health Network, Pittsburgh, PA, United States.
DOI:10.25259/SNI_311_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Stephen Jaffee1, Jyothika Mamadhi2, Trent Kite1, Dallas E. Kramer1, Nestor Tomycz1. Delayed epidural hematoma after spinal cord stimulator implantation in a patient with von Willebrand disease: Illustration. 06-Jun-2025;16:227
How to cite this URL: Stephen Jaffee1, Jyothika Mamadhi2, Trent Kite1, Dallas E. Kramer1, Nestor Tomycz1. Delayed epidural hematoma after spinal cord stimulator implantation in a patient with von Willebrand disease: Illustration. 06-Jun-2025;16:227. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13612
Abstract
Background: A patient with von Willebrand disease developed a delayed epidural hematoma originating from the tunneling tract and gluteal generator pocket following the placement of a thoracic spinal cord stimulator (SCS).
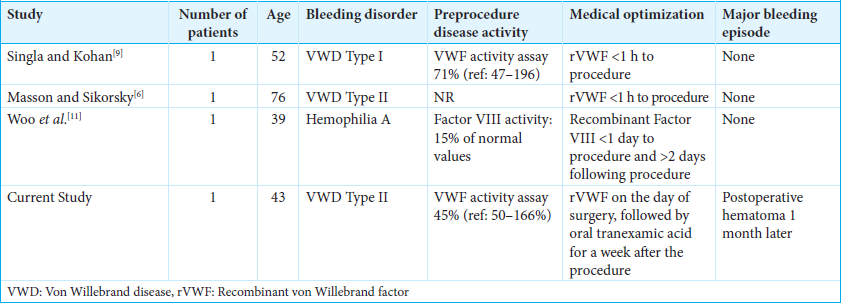
Case Description: A 43-year-old male with von Willebrand disease underwent thoracic SCS placement to treat chronic bilateral lower extremity pain with paresthesias in 2024. The patient had previously experienced a postoperative hematoma in 2010. At the time of the thoracic SCS placement, he received 7 days of prophylactic antihemophilic factor/von Willebrand factor complex therapy. One month following placement of the thoracic SCS, the patient noted significant swelling localized to the thoracic and buttock incisions. Exploratory surgery documented an additional hematoma tracking from the tunneling tract and gluteal generator pocket all the way into the epidural space; it was promptly removed. At the 6-month follow-up, he exhibited no further wounds or neurological issues.
Conclusion: Patients with coagulopathies, and critically, von Willebrand disease, undergoing SCS placement are at increased risk for postoperative hematomas.
Keywords: Coagulopathy, Spinal cord stimulation, Von Willebrand disease
INTRODUCTION
More spinal cord stimulators (SCSs) are being placed for treating various thoraco-lumbar pain syndromes.[
CASE DESCRIPTION
A 43-year-old male with von Willebrand disease type 2M originally presented with cervical myelopathy managed with multiple cervical procedures (i.e., anterior cervical discectomy/fusions at C6-C7 in 2010, C3-C5 in 2022, and C5-T1 in 2022). Following the original anterior cervical discectomy and fusion in 2010, he suddenly developed increased paraparesis attributed to acute postoperative hematoma within 24 h of surgery. The clot was removed, and he required 4 units of packed red blood cells in transfusion. Notably, in 2022, he required a T3-T9 laminectomy for thoracic myelopathy for residual pain. In 2024, he underwent elective placement of a thoracic SCS. Before this SCS surgery, his International Society on Thrombosis and Hemostasis bleeding assessment tool score was 7 (a score >5 is abnormal): his most recent von Willebrand factor activity level was 45% (reference range is 50–166% preferred), and factor VIII activity was 69% (reference range 50–150% is preferred). Preoperative platelet aggregation studies indicated prolonged closure time with collagen/adenosine diphosphate stimulation.
Pain management placed a percutaneous SCS trial that significantly improved his lower extremity (60–70%) and (20–30%) back pain; the permanent SCS was placed in October 2024. Prophylactically, he received antihemophilic factor/von Willebrand factor complex (50 units/kg twice daily for 3 days, followed by 50 units/kg daily for 4 days) to maintain von Willebrand factor ristocetin cofactor (VWF: Rco) and factor VIII (FVIII) trough levels above 50 units/dL, without exceeding 200 units/dL for VWF: Rco or 250 units/dL for FVIII.
Postoperative management
Following thoracic SCS implantation, he was given oral tranexamic acid (1.3 g 3 times daily) for 7 days. One month later, however, the patient developed significant increased swelling attributed to a postoperative hematoma at the thoracic and buttock incision sites; interestingly, he remained neurologically intact. Before repeated surgery for clot removal, he received an antihemophilic factor/von Willebrand factor complex infusion and tranexamic acid. At surgery, a large, coagulated hematoma originated from the tunneling tract of the gluteal pulse generator that extended into the epidural space, but paddle lead/anchoring sites remained intact. Examination of the gluteal incision revealed a coagulated hematoma without active hemorrhage. Postoperatively, the patient received an additional 7 days of antihemophilic factor/von Willebrand factor complex therapy. He was later discharged postoperative on day 3 without any residual hematoma and/or other adverse events.
DISCUSSION
This case highlights the risk of hemorrhage at sites beyond the epidural space, including the tunneling tract and implantable pulse generator pockets for patients with von Willebrand disease. Despite multidisciplinary perioperative optimization of the patient’s von Willebrand disease, delayed hematomas may occur. To avoid postoperative hematomas, patients with von Willebrand disease should preoperatively receive liberal administration of clotting factors and tranexamic acid along with vigilant monitoring for delayed hematomas.
Postoperative hematomas for SCS placement due to congenital bleeding disorders, including von Willebrand disease, hemophilia a and c
Other case reports demonstrate uncomplicated postoperative courses in patients with congenital bleeding (i.e., hemophilia A, C, or von Willebrand disease) disorders receiving SCS, where appropriate preoperative prophylaxis has been used [
CONCLUSION
Multidisciplinary teams should be involved when treating patients with von Willebrand disease undergoing SCS implants to avoid bleeding complications. When they occur, vigilant management protocols should be followed preoperatively, intraoperatively, and long-term postoperatively.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Deer TR, Narouze S, Provenzano DA, Pope JE, Falowski SM, Russo MA. The Neurostimulation Appropriateness Consensus Committee (NACC): Recommendations on Bleeding and Coagulation Management in Neurostimulation Devices. Neuromodulation. 2017. 20: 51-62
2. Federici AB. The factor VIII/von Willebrand factor complex: Basic and clinical issues. Haematologica. 2003. 88: Erep02
3. Luo H, Yan X, Ren Y, Zhang H, Pan W. The efficacy and safety of tranexamic acid in transforaminal lumbar interbody fusion: A systematic review and meta-analysis. EFORT Open Rev. 2023. 8: 919-25
4. Luo H, Yang Y, Wang Z, Ma L, Xie C. Efficacy and safety of tranexamic acid in cervical spine surgery: A systematic review and meta-analysis. Front Neurol. 2024. 15: 1405773
5. Ma J, Huang Z, Huang Q, Zhou Z, Pei F, Shen B. Tranexamic acid combined with compression dressing reduces blood loss in gluteal muscle contracture surgery. BMC Surg. 2022. 22: 46
6. Masson S, Sikorsky M. Spinal cord stimulator placement in a patient with type 2 von Willebrand’s disease. Interv Pain Manag Rep. 2018. 2: 153-5
7. Moeschler SM, Warner NS, Lamer TJ, Bendel MA, Warner MA, Eldrige JS. Bleeding complications in patients undergoing percutaneous spinal cord stimulator trials and implantations. Pain Med. 2016. 17: 2076-81
8. Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: Clinical efficacy and potential mechanisms. Pain Pract. 2018. 18: 1048-67
9. Singla P, Kohan LR. Spinal cord stimulator placement in patient with von Willebrand disease: A case report. A A Pract 2020. ;. 14: 149-51
10. Surgeons . Available from: https://www.aans.org/patients/conditions-treatments/spinal-cord-stimulation [Last accessed on 2025 Mar 10].
11. Woo S, Kim DH, Kim YC, Yoo Y. Successful spinal cord stimulator implantation in a patient with hemophilia A: A case report. Int J Pain. 2022. 13: 78-83
12. Yu N, Ross J, Romo J, Egan K, Pfeffer M. ID: 336917 spinal cord stimulation in a patient with hemophilia C. Neuromodulation. 2024. 27: S95