- Department of Neurosurgery, Saint Louis University School of Medicine, Saint Louis, Missouri, United States.
- Department of Neurosurgery, Texas Tech University Health Sciences Center, Lubbock, Texas, United States.
- Department of Neurosurgery, Saint Louis University, Saint Louis, Missouri, United States.
Correspondence Address:
Mayur S. Patel, Department of Neurosurgery, Saint Louis University School of Medicine, Saint Louis, Missouri, United States.
DOI:10.25259/SNI_1150_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mayur S. Patel1, Justin K. Zhang1, Ali Saif Raza Khan2, Georgios Alexopoulos1, Maheen Q. Khan3, Philippe J. Mercier1, Joanna M. Kemp1. Delayed peritoneal shunt catheter migration into the pulmonary artery with indolent thrombosis: A case report and narrative review. 04-Mar-2022;13:77
How to cite this URL: Mayur S. Patel1, Justin K. Zhang1, Ali Saif Raza Khan2, Georgios Alexopoulos1, Maheen Q. Khan3, Philippe J. Mercier1, Joanna M. Kemp1. Delayed peritoneal shunt catheter migration into the pulmonary artery with indolent thrombosis: A case report and narrative review. 04-Mar-2022;13:77. Available from: https://surgicalneurologyint.com/surgicalint-articles/11423/
Abstract
Background: Ventriculoperitoneal (VP) shunts are the preferred surgical treatment for hydrocephalus, and rarely, these operations may be complicated by catheter migration to ectopic sites. We present the case of an asymptomatic VP shunt patient with delayed peritoneal catheter migration into the pulmonary artery shunt catheter migration into the pulmonary artery (SCMPA) complicated by knotting and indolent thrombosis, necessitating open-heart surgery for system retrieval.
Methods: We conducted a literature review in PubMed, Scopus, and Web of Science of prior similar reported cases and present the results of 24 cases of SCMPA.
Results: An asymptomatic 12-year-old male presented with SCMPA noted on routine annual follow-up imaging. Preoperative CT angiogram indicated extensive catheter looping into the pulmonary artery without evidence of thrombosis. Less invasive attempts to retrieve the retained catheter were unsuccessful, and open-heart surgery was required. Intraoperatively, a nonocclusive pulmonary arterial thrombus surrounding the knotted catheter was discovered that was lysed successfully before system retrieval.
Conclusion: VP shunt catheter migration into the pulmonary artery (SCMPA) with concurrent large vessel thrombosis can develop in pediatric patients incidentally without any clinical symptoms. Our report suggests that preoperative CT angiogram may be insufficient to detect arterial thrombosis in the presence of extensive intravascular catheter knotting. An open-chest approach may be the only viable surgical option for catheter retrieval in the presence of complex catheter coiling. The use of anticoagulation following open-heart surgery for retrieval of a migrated VP shunt catheter remains unclear, we here propose that continuation of long-term therapeutic anticoagulation may successfully prevent thrombus relapse.
Keywords: Catheter migration, Hydrocephalus, Shunt, Shunt complication, Shunt migration
INTRODUCTION
Ventriculoperitoneal (VP) shunting is the most common treatment for the management of congenital hydrocephalus, with more than 30,000 systems placed annually in the United States.[
VP shunt complications include catheter obstruction, infection, system disconnection, hardware failure, and pseudocyst formation.[
A narrative literature review was performed in PubMed, Scopus, and Web of Science using the terms “ventriculoperitoneal shunt” and “migration” or “extrusion” or “misplacement.” After screening the databases and removing duplicate entries, a total of 33 cases of intracardiac VP shunt migration which include the 24 cases of SCMPA were identified [
CASE DESCRIPTION
A 12-year-old male with a history of open lumbosacral myelomeningocele treated with postnatal repair and Chiari-II-related shunted hydrocephalus presented to the neurosurgery clinic for his annual follow-up visit. The patient did not have any significant cardiac or pulmonary history. His initial shunt was placed at the age of 4 weeks, where a right VP system was utilized for permanent cerebrospinal fluid (CSF) diversion. The patient required a single revision at 11 years of age after presenting with incidental ventriculomegaly on routine follow-up imaging. He was found to have a disconnected distal VP catheter at the level of the neck. Only the distal portion of the VP system was revised, wherein the new peritoneal catheter was proximally connected to the existing shunt valve. The placement of the new catheter was uneventful; there was no copious tunneling during the procedure or damage to any adjacent structures of the neck or chest [
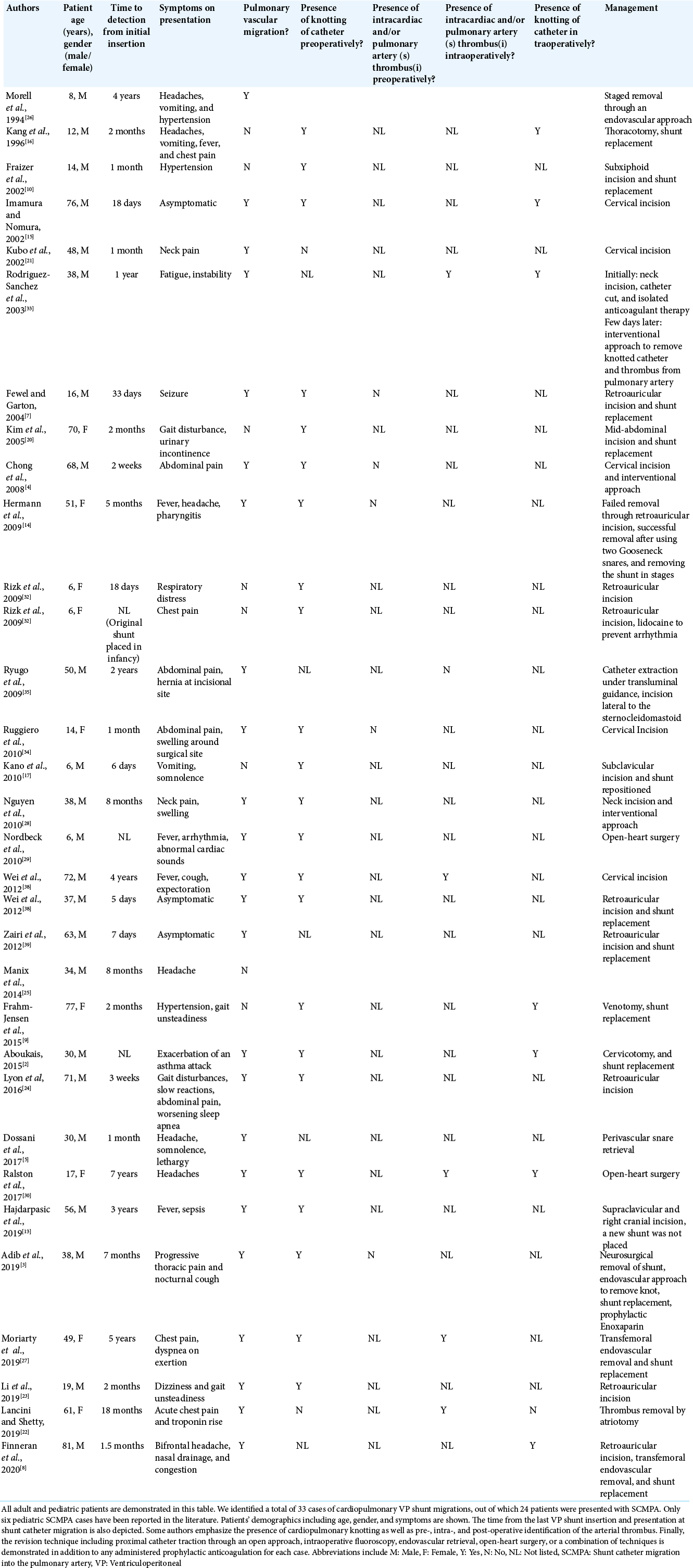
Figure 1:
X-ray shunt series completed on postoperative day 1 after the initial revision at 11 years of age and 1 year before distal shunt catheter migration. Chest and abdominal AP X-ray shunt series demonstrated peritoneal VP shunt catheter disconnection. The abandoned system on the right (red arrow) represents the old, disconnected shunt catheter while the distal catheter on the left (green arrow) represents the newly inserted peritoneal shunt system.
At 12 years of age, approximately 1 year following his single VP shunt revision, our patient was incidentally found with SCMPA on routine imaging during his regular follow-up clinic visit. On examination, the patient was asymptomatic with stable vital signs and denied any complaints at that time. Brain MRI revealed supratentorial ventriculomegaly, which was increased from baseline scans. Further workup with XR shunt series demonstrated distal shunt catheter migration into the thoracic cavity [
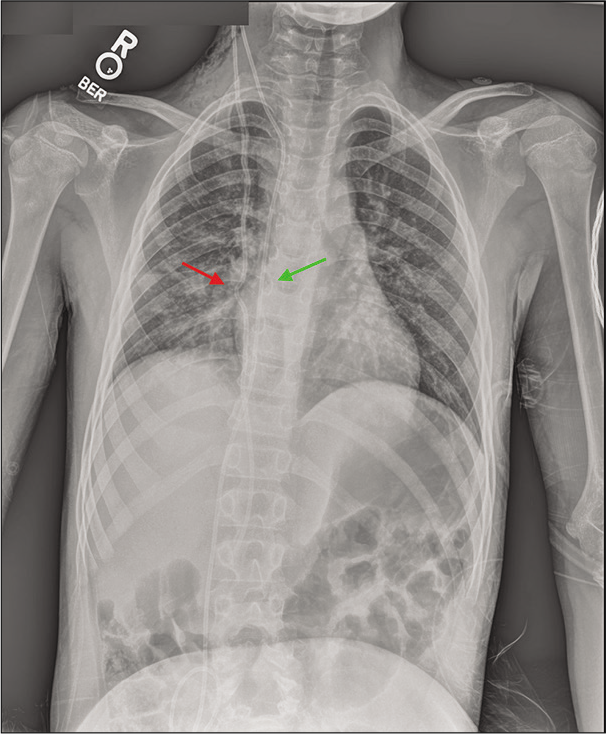
Figure 2:
T2 MRI axial views with contrast revealed ventriculomegaly (a). X-ray shunt series demonstrated migration of the distal VP shunt catheter into the heart and pulmonary artery, with evidence of intracardiac knotting (red arrow) (b). Noncontrast chest CT in the axial and sagittal views showed extensive knotting of the distal shunt catheter in the pulmonary vasculature (arrow) without definite arterial thrombosis (c and d).
Extensive discussions with the patient’s family regarding the extreme rarity of this condition were made, and we mutually agreed to proceed with surgical removal of the retained catheter to prevent any further cardiovascular complications. Initially, we attempted to remove the system through the existing retroauricular surgical incision, utilizing gentle traction. The migrated peritoneal catheter was identified at its proximal connection to the shunt valve, and after multiple traction attempts, we failed to obtain the system. We then proceeded with catheter manipulation under intraoperative fluoroscopy. Following multiple unsuccessful attempts, the patient developed premature ventricular contractions with any further catheter manipulation. Hence, a tortuous course into the pulmonary vasculature and/or extensive knotting was suspected. The interventional team was consulted, who stated that an endovascular approach for retrieval would not be feasible. It was soon made evident that the retained catheter could only be removed through an open-chest approach. After further discussions regarding the risks of abandoning the retained shunt catheter in the pulmonary vasculature, the patient was placed onto extracorporeal circulation and remained in the ICU for 48 h on therapeutic anticoagulation with IV heparin.
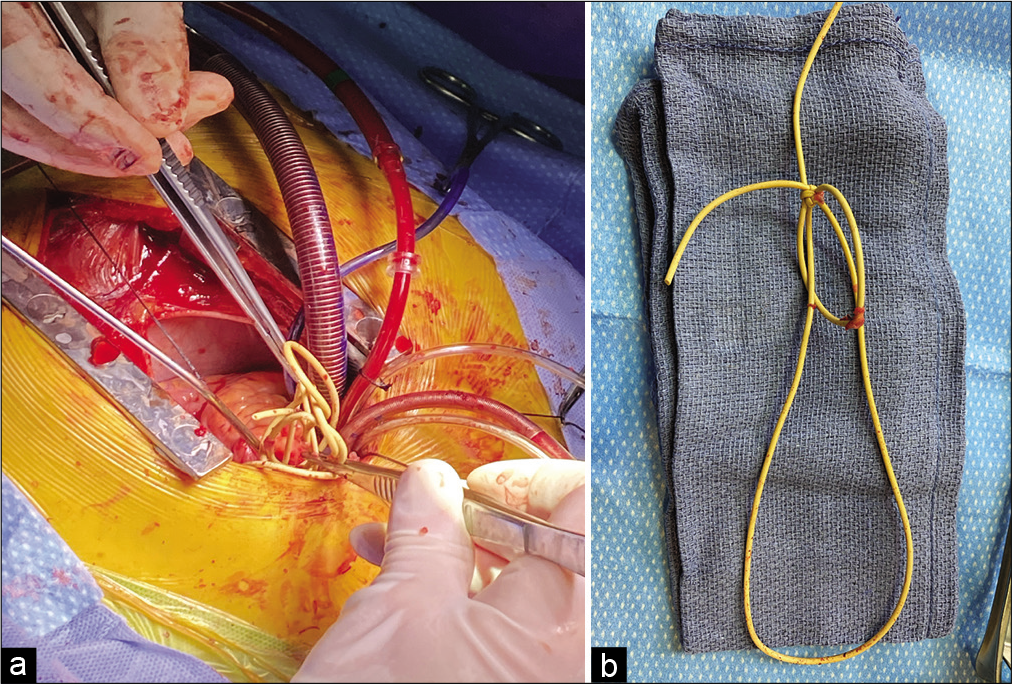
Our patient returned to the operating room on cardiopulmonary bypass approximately 48 h following the initial attempts for shunt retrieval. A midline sternal incision was made by our colleagues in cardiothoracic surgery. Temporary ligation of the pulmonary artery was performed using a purse string to obtain access to the migrated shunt catheter. A pulmonary arteriotomy was made, which revealed that the retained shunt catheter was coiled multiple times [
DISCUSSION
VP shunting is the first-line treatment of congenital hydrocephalus. Although an effective procedure, it is associated with failure rates up to 23% in the pediatric population.[
Historical background
VP shunt migration to ectopic sites is a relatively rare phenomenon in terms of shunt failure. The first cardiac migration of a distal shunt catheter was reported by Morell et al. in 1994 in a 12-year-old male. The patient presented with headaches, vomiting, and hypertension 4 years after the initial shunt placement. This was also the first reported case of shunt migration into the pulmonary vasculature.[
The first case of SCMPA in a pediatric patient who underwent an open-heart surgery was reported by Kang et al. in 1996. This patient was also symptomatic and presented 2 months after the initial shunt placement. Interestingly, the authors also observed intraoperative knotting of the distal catheter in the right ventricle. This patient had a pure cardiac migration without involvement of the pulmonary arteries.[
Clinical presentation
SCMPA is a rarer cause of cardiopulmonary shunt migration, where the catheter migrates to the pulmonary vasculature.[
Diagnosis, management options, and outcomes
There are no clear guidelines for the diagnosis of shunt migration and SCMPA.[
Various surgical interventions exist to manage VP shunt migration. Minor surgery with posterior auricular or cervical incisions in adjunction with cardiovascular surgery has been reported.[
Data on long-term outcomes for VP shunt revisions and replacements are limited.[
Due to the high rate of VP shunt failures, especially in pediatric patients and regardless of revisions, it is recommended that patients are followed at least through the transition to adulthood as revisions and replacements are reported in the literature after 17 years of initial shunt placement.[
Literature review
Delayed SCMPA resulting in arterial thrombosis following VP shunt insertion is an extremely rare complication, with 24 published reports among the adult and pediatric population [
Mechanism of shunt migration
Cardiopulmonary VP shunt catheter migration has been generally associated with two different proposed mechanisms in the literature.[
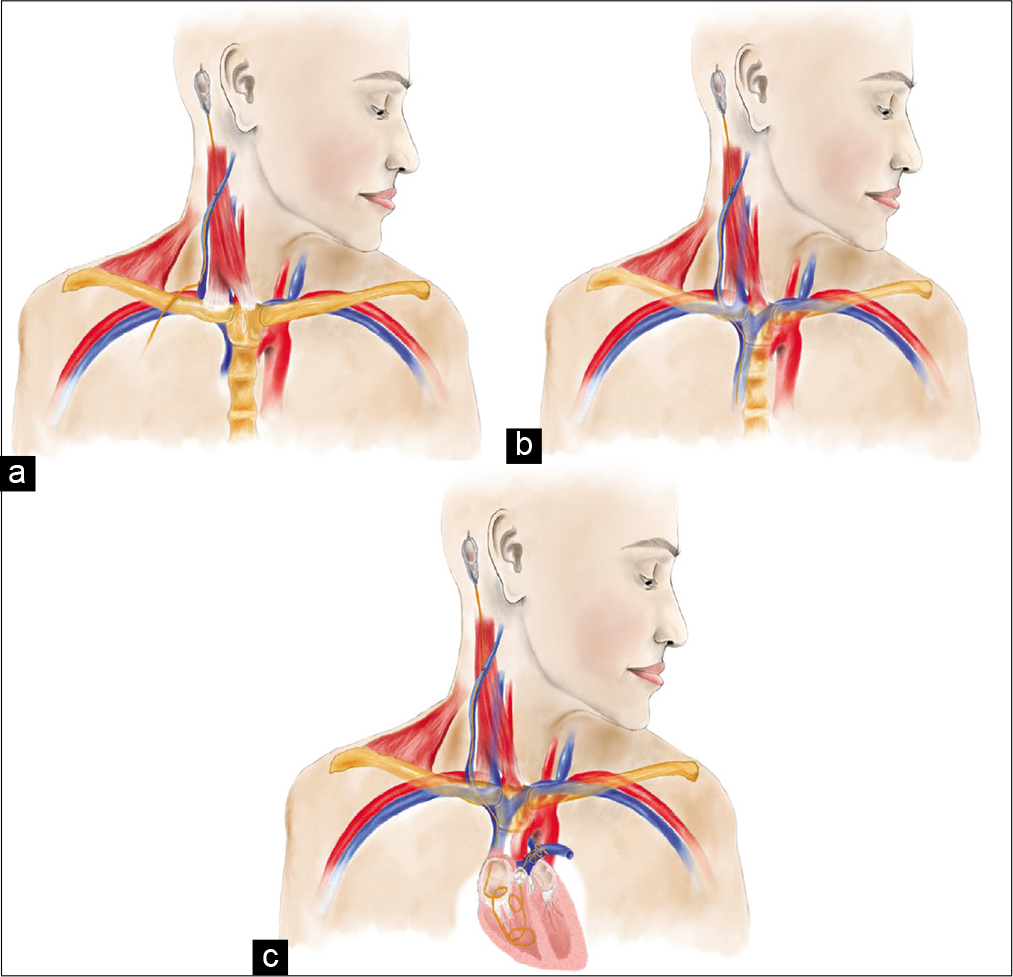
Figure 4:
Medical illustrations describing proposed mechanisms of VP shunt migration. Erosion of the external jugular vein due to excessive traction form neck movements at varying levels of the vein (a). Iatrogenic perforation of the external jugular vein during tunneling of the distal catheter (b). In both (a and b), negative intrathoracic pressure along with venous flow further displaces the catheter into the vein, pulling it caudally. If the catheter migrates far enough, it can be lodged and knotted in the pulmonary arteries, as indicated in caption (c).
Surgical management of SCMPA
Surgical strategies to correct SCMPA range from open revision surgery with catheter manipulation using gentle traction at the most proximal site to endovascular retrieval using loop snare device.[
Among the six SCMPA pediatric reports, the catheter was retrieved through an open cardiac approach in 3 cases (50%), while two patients, as reported by Kang et al. and Ralston et al.,[
Concurrent pulmonary arterial thrombosis
To the authors’ knowledge, no study has noted the presence of pulmonary thromboses on preoperative imaging in the setting of SCMPA.[
The need for continuation of therapeutic anticoagulation after clot removal remains unclear. If detected intraoperatively, it can be managed medically through the same guidelines as central venous catheter-related thrombosis.[
CONCLUSION
SCMPA with concurrent large vessel thrombosis can develop incidentally in pediatric patients without any clinical symptoms. Preoperative CT angiogram may be insufficient to detect arterial thrombosis in the presence of extensive catheter knotting. Clinicians should be aware of the limitations of preoperative imaging and understand that the lack of symptoms may not necessarily be an optimal indicator of the extent of concurrent thrombosis. An open-chest approach may be the only viable surgical option for catheter retrieval in the presence of complex intravascular catheter coiling. Continuation of long-term therapeutic anticoagulation in these patients may successfully prevent arterial thrombus relapse.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abode-Iyamah KO, Khanna R, Rasmussen ZD, Flouty O, Dahdaleh NS, Greenlee J. Risk factors associated with distal catheter migration following ventriculoperitoneal shunt placement. J Clin Neurosci. 2016. 25: 46-9
2. Aboukais R, Zairi F, Marinho P, Lejeune JP. Management of cardiac migration of a distal shunt catheter: The radiological pitfalls. Neurochirurgie. 2015. 61: 43-5
3. Adib SD, Lescan M, Renovanz M, Schuhmann MU, Trakolis L, Bongers M. Intracardial catheter migration of a ventriculoperitoneal shunt: Pathophysiology and interdisciplinary management. World Neurosurg. 2020. 135: 222-7
4. Chong JY, Kim JM, Cho DC, Kim CH. Upward migration of distal ventriculoperitoneal shunt catheter into the heart: Case report. J Korean Neurosurg Soc. 2008. 44: 170-3
5. Dossani RH, Maiti TK, Patra DP, Nanda A, Cuellar H. endovascular retrieval of migrated distal end of ventriculoperitoneal shunt from bilateral pulmonary arteries: A technical note. Ann Vasc Surg. 2017. 45: 305-14
6. ElSankari S, Baledent O, van Pesch V, Sindic C, de Broqueville Q, Duprez T. Concomitant analysis of arterial, venous, and CSF flows using phase-contrast MRI: A quantitative comparison between MS patients and healthy controls. J Cereb Blood Flow Metab. 2013. 33: 1314-21
7. Fewel ME, Garton HJ. Migration of distal ventriculoperitoneal shunt catheter into the heart. Case report and review of the literature. J Neurosurg. 2004. 100: 206-11
8. Finneran MM, Nardone E, Marotta DA, Smith GB, Gordhan A. Spontaneous migration of a ventriculoperitoneal shunt into the venous system: A multidisciplinary approach. Cureus. 2020. 12: e7779
9. Frahm-Jensen G, Newton PR, Drummond KJ, Wagner TP, Mees BM. Intracardiac migration and knotting of a ventriculoperitoneal shunt. J Clin Neurosci. 2015. 22: 771-3
10. Frazier JL, Wang PP, Patel SH, Benson JE, Cameron DE, Hoon AH. Unusual migration of the distal catheter of a ventriculoperitoneal shunt into the heart: Case report. Neurosurgery. 2002. 51: 819-22
11. Ghritlaharey RK. ventriculoperitoneal shunt disconnection, shunt migration, and silent bowel perforation in a 10-year-old boy. J Neurosci Rural Pract. 2019. 10: 342-5
12. Gonzalez DO, Mahida JB, Asti L, Ambeba EJ, Kenney B, Governale L. Predictors of ventriculoperitoneal shunt failure in children undergoing initial placement or revision. Pediatr Neurosurg. 2017. 52: 6-12
13. Hajdarpasic E, Dzurlic A, Mahmutbegovic N, Zahirovic S, Ahmetspahic A, Arnautovic K. Sepsis caused by bacterial colonization of migrated distal ventriculoperitoneal shunt catheter into the pulmonary artery: A first case report and literature review. World Neurosurg. 2019. 126: 172-80
14. Hermann EJ, Zimmermann M, Marquardt G. Ventriculoperitoneal shunt migration into the pulmonary artery. Acta Neurochir (Wien). 2009. 151: 647-52
15. Imamura H, Nomura M. Migration of ventriculoperitoneal shunt into the heart--case report. Neurol Med Chir (Tokyo). 2002. 42: 181-3
16. Kang JK, Jeun SS, Chung DS, Lee IW, Sung WH. Unusual proximal migration of ventriculoperitoneal shunt into the heart. Childs Nerv Syst. 1996. 12: 176-9
17. Kano T, Kurosaki S, Iwasa S, Wada H. Migration of a distal ventriculoperitoneal shunt catheter into the internal jugular vein and heart through the external jugular vein: Case report. Neurol Med Chir (Tokyo). 2010. 50: 945-8
18. Khan F, Rehman A, Shamim MS, Bari ME. Factors affecting ventriculoperitoneal shunt survival in adult patients. Surg Neurol Int. 2015. 6: 25
19. Khan F, Shamim MS, Rehman A, Bari ME. Analysis of factors affecting ventriculoperitoneal shunt survival in pediatric patients. Childs Nerv Syst. 2013. 29: 791-802
20. Kim MS, Oh CW, Hur JW, Lee JW, Lee HK. Migration of the distal catheter of a ventriculoperitoneal shunt into the heart: Case report. Surg Neurol. 2005. 63: 185-7
21. Kubo S, Takimoto H, Takakura S, Iwaisako K, Yamanaka K, Hosoi K. Peritoneal shunt migration into the pulmonary artery--case report. Neurol Med Chir (Tokyo). 2002. 42: 572-4
22. Lancini D, Shetty R. Spontaneous transcardiac migration of a ventriculoperitoneal shunt. Eur Heart J. 2020. 41: 3015
23. Li W, Li Y, Sun Y, Chen L. Migration of a distal ventriculoperitoneal shunt catheter into the pulmonary vasculature: A report of an unusual case and a review of the literature. J Craniofac Surg. 2019. 30: e243-4
24. Lyon K, Ban VS, Bedros N, Aoun SG, El Ahmadieh TY, White J. Migration of a ventriculoperitoneal shunt into the pulmonary vasculature: Case report, review of the literature, and surgical pearls. World Neurosurg. 2016. 92: 585.e5-e11
25. Manix M, Sin A, Nanda A. Distal ventriculoperitoneal shunt catheter migration to the right ventricle of the heart--a case report. J La State Med Soc. 2014. 166: 21-5
26. Morell RC, Bell WO, Hertz GE, D’Souza V. Migration of a ventriculoperitoneal shunt into the pulmonary artery. J Neurosurg Anesthesiol. 1994. 6: 132-4
27. Moriarty JM, Quesada CJ, Wang AC, Yang EH. Knot in the right place. J Vasc Interv Radiol. 2019. 30: 249
28. Nguyen HS, Turner M, Butty SD, Cohen-Gadol AA. Migration of a distal shunt catheter into the heart and pulmonary artery: Report of a case and review of the literature. Childs Nerv Syst. 2010. 26: 1113-6
29. Nordbeck P, Beer M, Wirbelauer J, Ritter O, Weidemann F, Ertl G. Intracardial dislocation of a cranio-peritoneal shunt in a 6-year-old boy. Clin Res Cardiol. 2010. 99: 677-8
30. Ralston A, Johnson A, Ziemer G, Frim DM. Transcardiac migration of ventriculoperitoneal shunt requiring open cardiac surgery: Case report and review of the literature. Childs Nerv Syst. 2017. 33: 703-7
31. Reddy GK, Bollam P, Caldito G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 2014. 81: 404-10
32. Rizk E, Dias MS, Verbrugge J, Boop FA. Intracardiac migration of a distal shunt catheter: An unusual complication of ventricular shunts. Report of 2 cases. J Neurosurg Pediatr. 2009. 3: 525-8
33. Rodriguez-Sanchez JA, Cabezudo-Artero JM, Porras Estrada LF. Unusual migration of the distal catheter of a ventriculoperitoneal shunt into the heart: Case report. Neurosurgery. 2003. 52: 1510
34. Ruggiero C, Spennato P, De Paulis D, Aliberti F, Cinalli G. Intracardiac migration of the distal catheter of ventriculoperitoneal shunt: A case report. Childs Nerv Syst. 2010. 26: 957-62
35. Ryugo M, Imagawa H, Nagashima M, Shikata F, Hashimoto N, Kawachi K. Migration of distal ventriculoperitoneal shunt catheter into the pulmonary artery. Ann Vasc Dis. 2009. 2: 51-3
36. Soler GJ, Bao M, Jaiswal D, Zaveri HP, DiLuna ML, Grant RA. A review of cerebral shunts, current technologies, and future endeavors. Yale J Biol Med. 2018. 91: 313-21
37. Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ. Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J Neurosurg Pediatr. 2013. 11: 15-9
38. Wei Q, Qi S, Peng Y, Fan J, Lu Y. Unusual complications and mechanism: Migration of the distal catheter into the heart--report of two cases and review of the literature. Childs Nerv Syst. 2012. 28: 1959-64
39. Zairi F, du Moulinet d’Hardemaere V, Assaker R. Early cardiac migration of distal shunt catheter. Br J Neurosurg. 2012. 26: 545-6
40. Zhang H, Zhang J, Peng J, Hao X, Li G. The diagnosis of ventriculoperitoneal shunt malfunction by using phase-contrast cine magnetic resonance imaging. J Clin Neurosci. 2019. 64: 141-4