- Department of Medicine, Tiradentes University, Aracaju, Brazil
- Department of Medicine, Federal University of Sergipe, Aracaju, Brazil
- Health Sciences Graduate Program, Federal University of Sergipe, Aracaju, Brazil
Correspondence Address:
Bruno Fernandes de Oliveira Santos, Health Sciences Graduate Program, Federal University of Sergipe, Aracaju, Brazil.
DOI:10.25259/SNI_924_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryan Fernando Menezes, Rafael Lisboa Prudente1, Erom Lucas Alves Freitas2, Bruno Fernandes de Oliveira Santos3. Dentato-rubro-thalamic tract tractography: Constrained spherical deconvolution versus diffusion tensor imaging for essential tremor deep brain stimulation targeting. 30-May-2025;16:211
How to cite this URL: Ryan Fernando Menezes, Rafael Lisboa Prudente1, Erom Lucas Alves Freitas2, Bruno Fernandes de Oliveira Santos3. Dentato-rubro-thalamic tract tractography: Constrained spherical deconvolution versus diffusion tensor imaging for essential tremor deep brain stimulation targeting. 30-May-2025;16:211. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13592
Abstract
Background: This study aimed to compare diffusion tensor imaging (DTI) and constrained spherical deconvolution (CSD) techniques in the segmentation of the dentate-rubro-thalamic tract (DRTT), evaluating their anatomical accuracy and applicability for surgical planning in deep brain stimulation (DBS) for essential tremor surgery in two patients.
Methods: The images were acquired using two 1.5 Tesla magnetic resonance imaging protocols optimized for both DTI and CSD. Preprocessing included noise removal, artifact correction, and distortion adjustments. Segmentation was performed using region-of-interest definitions from specific atlases. CSD was applied with response function estimation, followed by fiber orientation reconstruction and tracking using the probabilistic improved fiber orientation distribution 2(iFOD2) algorithm. The DTI technique was conducted with tensor calculation and fractional anisotropy (FA) analysis. Volume and FA metrics were compared between techniques to evaluate segmentation accuracy for the DRTT.
Results: CSD-based segmentation showed significantly larger volumes in the left hemisphere compared to DTI, along with higher FA values. In the right hemisphere, the difference was not statistically significant. Dice similarity index analysis revealed very low correspondence between the techniques in both hemispheres, suggesting greater precision of CSD in DRTT segmentation.
Conclusion: CSD proved to be more effective in DRTT segmentation, with better angular resolution and greater detailing of axonal trajectories, especially in regions with fiber crossing.
Keywords: Constrained spherical deconvolution, Deep brain stimulation, Diffusion tensor imaging, Essential tremor, Tractography
INTRODUCTION
Essential tremor (ET) is a common neurological disorder characterized by action and postural tremors. The dentato-rubro-thalamic tract (DRTT) has been identified as a crucial white matter pathway involved in the circuits implicated in ET. Deep brain stimulation (DBS) targeting this tract has shown promising results in treating refractory cases of ET.[
Among the methods for performing tractography, diffusion tensor imaging (DTI) is widely used due to its ability to map the fractional anisotropy (FA) of white matter fibers.[
To overcome these limitations, advanced techniques like constrained spherical deconvolution (CSD) have been developed. CSD allows for the reconstruction of the fiber orientation distribution function (fODF), providing a more precise and detailed visualization of the multidirectionality of fibers within a voxel.[
The ventral intermediate nucleus (VIM) of the thalamus is the classic target in DBS for treating ET, generally determined by indirect targeting (commissural coordinates).[
MATERIALS AND METHODS
Image acquisition
Forty diffusion images at 7 Tesla resolutions from the Human Connectome Project (HCP) database were evaluated. The acquisition protocol for these images was as follows: repetition time (TR) of 7000 ms, echo time (TE) of 71.2 ms, b-values of 1000 and 2000 s/mm2, and an echo spacing of 0.82 ms. Structural T1-weighted images were obtained using Siemens devices. The age range of the subjects was from 22 to 35 years, comprising 29 women and 11 men. All subjects had no brain pathology.
For the two clinical cases, the diffusion image acquisition protocol was carried out using two different 1.5 Tesla magnetic resonance imaging (MRI) systems. This dual-protocol approach was employed to ensure compatibility with different clinical settings and to validate the robustness of our methodology across different imaging parameters. Both clinical protocols were optimized to ensure adequate resolution and compatibility with the application CSD techniques, ensuring the necessary quality for subsequent tractographic analysis. In the first clinical case, the acquisition was performed on a gradient echo (GE) scanner, where diffusion sequences were obtained with the following parameters: TR = 8,325 ms, TE = 0.1099 ms, acquisition matrix of 128 × 128, field of view (FOV) of 640 mm, slice thickness of 5 mm, and gradient with 33 directions. T1-weighted structural images were acquired with TR = 0.0086 ms, TE = 0.0034 ms, a 256 × 256 matrix, and a FOV of 256 mm. In the second clinical case, diffusion images were acquired with a Philips 1.5 Tesla scanner, using a sequence with TR = 3,291 ms, TE = 0.0934 ms, acquisition matrix of 90 × 90, FOV of 225 mm, slice thickness of 2.5 mm, and a gradient of 32 directions. The T1-weighted sequence was acquired with TR = 0.0088 ms, TE = 0.0041 ms, a 240 × 240 matrix, and a FOV of 120 mm.
Pre-processing
The diffusion images were subjected to pre-processing steps to enable DRTT tractographic segmentation. First, signal noise was removed using the dwidenoise tool, followed by the use of the mrdegibbs tool to eliminate potential acquisition artifacts. In addition, the images were processed with the help of the dwifslpreproc tool, which performs a pipeline for correcting distortions caused by susceptibility and motion.
The images from the HCP underwent preprocessing steps in their acquisition protocol, which included motion correction and distortion correction. Therefore, it was not necessary to subject this group of images to the preprocessing typically required for clinical images.
Definition of regions of interest (ROIs)
Tractography was performed using a ROI-based approach, with ROIs derived from atlases and used as targets for DRTT segmentation. For the inclusion of ROIs, the dentate nucleus of the cerebellum, defined by the Spatially Unbiased Atlas Template of the cerebellum and brainstem, was used as the seed region to initiate segmentation. In addition, contralateral hemisphere inclusion masks were selected to segment the DRTT as follows: The contralateral red nucleus, defined by the Parkinson’s Disease 25 subjects histological atlas (PD25), the contralateral ventrolateral thalamic nucleus (PD25), and the contralateral primary motor cortex defined by the Juelich Cytoarchitectonic Atlas.
For exclusion ROIs, to remove the ipsilateral fibers of the dentate nucleus, the following masks were selected: the corpus callosum defined by the Johns Hopkins University International Consortium of Brain Mapping-DTI-81 white matter atlas, the corresponding bulb of the brainstem (manually defined with the assistance of the Harvard-Oxford Subcortical Structural Atlas), the ipsilateral ventrolateral thalamic nucleus, and the ipsilateral red nucleus. All ROIs were originally defined in the common space of the Montreal Neuroscience Institute. To be used in tractography directly within the native diffusion space of each patient, we performed diffeomorphic non-linear co-registration using the Advanced Normalization Tools (ANTs) software package.
CSD technique
The CSD technique was implemented using the MRtrix3 software package[
After the reconstruction of the fODFs, tractography was conducted using the probabilistic iFOD2 algorithm. This method was chosen for its ability to handle the complexity of fiber trajectories in regions with crossing or dispersing directions. The iFOD2 algorithm is particularly effective in resolving multiple fiber populations within a voxel, making it ideal for tracking complex white matter structures like the DRTT. Fiber tracking was parameterized to generate a total of 5,000 streamlines, trials 2000, max_attempts_per_seed 5000, angle 45°, and max_length 10000, ensuring a comprehensive representation of neural connections.
Finally, the obtained segmentation was subjected to cross-space registration using a diffeomorphic non-linear co-registration method through the ANTs software. This process was performed to align the segmented diffusion images with the anatomical space of each patient’s T1-weighted sequences, ensuring spatial conformity between the structures of interest and surrounding areas [
DTI method
A deterministic tractography based on DTI was performed to compare with CSD-based tractography. For this purpose, the Tensor_Det deterministic algorithm from the MRtrix3 toolkit was used. In this approach, the dentate nucleus, the contralateral red nucleus, and the contralateral ventrolateral nucleus were defined as seed ROIs, while the corpus callosum, the contralateral dentate nucleus, and the ipsilateral red nucleus were set as exclusion ROIs. All ROIs were defined according to the atlases used for CSD tractography. The parameters were set with -maxlen 10000, -angle 45, and -seeds 2000000. All other tool options were left at default settings.
Stereotactic method
The stereotactic targeting methodology utilized the Eximius Med Software Stereotactic Module (Artis, Brasilia, Brazil). High-resolution MRI scans were acquired, and the anterior commissure - posterior commissure (AC-PC) plane was defined. These images were then co-registered with computed tomography CT scans taken with the stereotactic frame in place. A standardized human brain stereotactic atlas was overlaid onto the co-registered images to aid in identifying subcortical structures. The intersection between the VIM of the Thalamus and the DRTT was identified as the primary target for electrode placement. Cartesian coordinates (x, y, and z) of the target were calculated relative to the mid-commissural point and compared with standard VIM target coordinates.[
RESULTS
CSD versus DTI
The DRTT segmentation results from the HCP cases using CSD and DTI techniques revealed significant differences in volume and FA metrics, particularly in the left hemisphere. The CSD technique showed a significantly larger segmented volume (10,230 mm3) compared to DTI (3,708 mm3; P = 0.0035), along with higher FA values (0.362 vs. 0.242; P < 0.0001) [
The similarity analysis between the volumes segmented by both techniques, measured by the Dice similarity index, showed low concordance in both hemispheres (0.013 on the left and 0.035 on the right). These values indicate a significant discrepancy between the techniques in DRTT delineation, highlighting the presumed superiority of CSD in tract reconstruction compared to DTI, which demonstrated limitations in identifying more complex axonal trajectories. Clinically, these findings suggest that CSD offers greater precision and detail in the segmentation of DRTT.
Clinical cases
Two patients, a 67-year-old man and a 70-year-old woman, both with action and postural tremors refractory to medical treatment (optimized doses of propranolol and primidone), underwent surgical treatment with DBS implantation targeting the DRTT. Instead of using the classic stereotactic coordinates for target definition in the VIM of the thalamus (VIM), we employed a direct targeting technique to position the electrodes along the DRTT trajectory that passes ventrally to the thalamus [
After electrode implantation, intraoperative tests (macro-stimulation) were performed, resulting in tremor control at low stimulation amplitudes (<1 mA) and a broad therapeutic window (>3 mA) in both cases. Postoperatively, both patients demonstrated sustained tremor control without adverse effects or surgical complications.
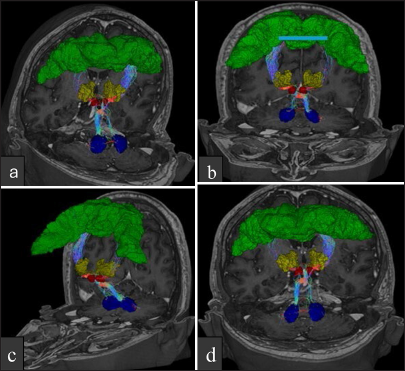
Figure 3:
Multiplanar coronal, sagittal, and axial views showing the position of the electrode in the thalamic target tract for DBS in patient 1. Tractography demonstrates the surgical accuracy of electrode placement. (a) Coronal view displaying electrode position within the thalamic target.(b)Sagittal view highlighting the electrode trajectory. (c) Axial view showing cross-sectional electrode localization. (d) 3D tractography confirming the accuracy of electrode positioning relative to thalamic structures.
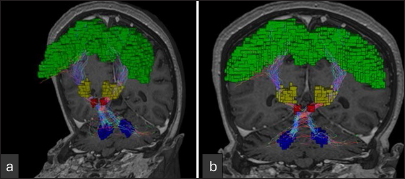
Figure 4:
Multiplanar reconstruction demonstrating the electrode trajectory in the thalamic target region for DBS in patient 2. (a) Coronal view showing electrode placement within the thalamic area. (b) Sagittal view depicting electrode trajectory and alignment. (c) Axial view with crosshair indicating electrode location. (d) 3D reconstruction illustrating stereotactic coordinates and vector direction of electrode insertion.
DISCUSSION
The comparison between DTI and CSD suggests the superiority of CSD in DRTT segmentation and three-dimensional reconstruction. CSD not only showed significantly larger segmented volumes and higher FA values, especially in the left hemisphere, but also demonstrated greater precision in trajectory delineation, facilitating the anatomical identification of the VIM of the thalamus, which is the primary therapeutic target for DBS. In contrast, DTI systematically underestimated the volume and complexity of the DRTT, generating less detailed and potentially inadequate results for precise surgical planning.
The DRTT plays a central role in modulating tremors and is one of the most important targets for neuromodulation in DBS procedures for treating ET.[
Anatomically, the DRTT originates in the superior cerebellar peduncle, decussates, and projects anteriorly to the red nucleus before reaching the ventral thalamus, located in proximity to the medial lemniscus (LM).[
Although the use of the DRTT in DBS planning for ET is not yet universally accepted, clinical studies suggest that this tract can play a central role in effectively treating tremors. Studies by Parras et al.[
The Dice similarity index between the two techniques highlighted this discrepancy, showing extremely low values for the correspondence between the segmented volumes (0.013 on the left and 0.035 on the right), suggesting that the representations obtained by DTI are insufficiently concordant with those generated by CSD. This difference reflects the limitation of DTI in identifying axonal trajectories in regions of complex crossing and curvature, underscoring the need for a more robust methodology, such as CSD, to ensure accurate tract visualization.[
In contrast, the CSD technique addresses these deficiencies by calculating the fODF distribution function, allowing for the precise identification of multiple bundle orientations within the same voxel. Using more detailed angular information and a deconvolution model that more accurately separates the directions of the bundles, CSD can segment the DRTT along its entire length, even in densely crossing regions, such as its anterior projection to the red nucleus and its entry into the ventral thalamus.[
Direct visualization of the VIM is insufficient in conventional imaging. Patients with anatomical variations benefit from the reconstruction of the DRTT through tractography techniques, such as DTI or CSD, as this fiber bundle can serve as a reference structure to guide the insertion of electrodes.[
Besides its applicability in treating ET, CSD shows great potential in other neurological conditions that require precise segmentation and accurate fiber visualization, such as Parkinson’s disease, defining boundaries for glioma resection, and assessing compromised tracts in ischemic lesions.[
However, despite promising results, challenges associated with the use of CSD remain. These include variability between different reconstruction software, lack of standardization in applied quantitative metrics, and high computational demands that are not feasible on most current hardware. This heterogeneity can result in variations in outcomes, necessitating more comparative studies and clinical trials to validate CSD’s superiority in different clinical scenarios and with larger populations. Until more evidence is consolidated, DTI remains the standard technique due to its simplicity and wide availability. Nonetheless, CSD stands out as an emerging methodology, offering a more robust and detailed resolution for segmenting complex tracts. It shows great potential to become the method of choice for planning neurosurgical interventions in anatomically complex regions, particularly as computational capabilities advance.[
As further studies consolidate these findings, CSD has the potential to become the preferred technique for tractography and planning neurosurgical interventions, such as DBS in patients with ET, gradually replacing DTI as the reference method in this context. These advancements in imaging and targeting techniques promise to significantly improve outcomes in functional neurosurgery, particularly in the treatment of movement disorders.
CONCLUSION
A comparative analysis between DTI and CSD techniques in DRTT segmentation suggests that CSD provides superior detailing of axonal trajectories, offering more precise reference points for VIM localization. The results indicate that CSD is capable of accurately delineating the anatomy of the tract, potentially enhancing surgical precision.
Ethical approval:
The research/study approved by the Institutional Review Board at UNIVERSIDADE FEDERAL DE SERGIPE – UFS, number 77903624.0.0000.5546, dated May 21, 2024.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirms that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Coenen VA, Sajonz B, Prokop T, Reisert M, Piroth T, Urbach H. The dentato-rubro-thalamic tract as the potential common deep brain stimulation target for tremor of various origin: An observational case series. Acta Neurochir (Wien). 2020. 162: 1053-66
2. Farquharson S, Tournier JD, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD. White matter fiber tractography: Why we need to move beyond DTI. J Neurosurg. 2013. 118: 1367-77
3. Freitas EL, De Oliveira Santos BF. Artificial intelligence in corticospinal tract segmentation using constrained spherical deconvolution. Surg Neurol Int. 2025. 16: 32
4. Lehman VT, Lee KH, Klassen BT, Blezek DJ, Goyal A, Shah BR. MRI and tractography techniques to localize the ventral intermediate nucleus and dentatorubrothalamic tract for deep brain stimulation and MR-guided focused ultrasound: A narrative review and update. Neurosurg Focus. 2020. 49: E8
5. Low HL, Ismail MN, Taqvi A, Deeb J, Fuller C, Misbahuddin A. Comparison of posterior subthalamic area deep brain stimulation for tremor using conventional landmarks versus directly targeting the dentatorubrothalamic tract with tractography. Clin Neurol Neurosurg. 2019. 185: 105466
6. Middlebrooks EH, Holanda VM, Tuna IS, Deshpande HD, Bredel M, Almeida L. A method for pre-operative single-subject thalamic segmentation based on probabilistic tractography for essential tremor deep brain stimulation. Neuroradiology. 2018. 60: 303-9
7. Mollink J, Van Baarsen KM, Dederen PJ, Foxley S, Miller KL, Jbabdi S. Dentatorubrothalamic tract localization with postmortem MR diffusion tractography compared to histological 3D reconstruction. Brain Struct Funct. 2016. 221: 3487-501
8. Morrison MA, Lee AT, Martin AJ, Dietiker C, Brown EG, Wang DD. DBS targeting for essential tremor using intersectional dentato-rubro-thalamic tractography and direct proton density visualization of the VIM: Technical note on 2 cases. J Neurosurg. 2021. 135: 806-14
9. Nowacki A, Schlaier J, Debove I, Pollo C. Validation of diffusion tensor imaging tractography to visualize the dentatorubrothalamic tract for surgical planning. J Neurosurg. 2018. 130: 99-108
10. Parras O, Domínguez P, Tomás-Biosca A, Guridi J. The role of tractography in the localisation of the Vim nucleus of the thalamus and the dentatorubrothalamic tract for the treatment of tremor. Neurología (Engl Ed). 2022. 37: 691-9
11. Pozzilli V, Marano M, Magliozzi A, Mallio CA, Marruzzo D, Barbieri FR. Deep brain stimulation of the dentato-rubrothalamic tract in a case of Holmes tremor: A constrained spherical deconvolution (CSD)-guided procedure. Neurol Sci. 2023. 44: 411-5
12. Rodrigues NB, Mithani K, Meng Y, Lipsman N, Hamani C. The emerging role of tractography in deep brain stimulation: Basic principles and current applications. Brain Sci. 2018. 8: 23
13. Sasada S, Agari T, Sasaki T, Kondo A, Shinko A, Wakamori T. Efficacy of fiber tractography in the stereotactic surgery of the thalamus for patients with essential tremor. Neurol Med Chir (Tokyo). 2017. 57: 392-401
14. Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004. 23: 1176-85
15. Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019. 202: 116137
16. Wong JK, Hess CW, Almeida L, Middlebrooks EH, Christou EA, Patrick EE. Deep brain stimulation in essential tremor: Targets, technology, and a comprehensive review of clinical outcomes. Expert Rev Neurother. 2020. 20: 319-31