- Department of Anesthesiology and Perioperative Medicine, University of California Los Angeles, UCLA, Los Angeles, California, USA
Correspondence Address:
Barbara Van de Wiele
Department of Anesthesiology and Perioperative Medicine, University of California Los Angeles, UCLA, Los Angeles, California, USA
DOI:10.4103/sni.sni_301_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Susana Vacas, Barbara Van de Wiele. Designing a pain management protocol for craniotomy: A narrative review and consideration of promising practices. 06-Dec-2017;8:291
How to cite this URL: Susana Vacas, Barbara Van de Wiele. Designing a pain management protocol for craniotomy: A narrative review and consideration of promising practices. 06-Dec-2017;8:291. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8692
Abstract
Background:Craniotomy is a relatively common surgical procedure with a high incidence of postoperative pain. Development of standardized pain management and enhanced recovery after surgery (ERAS) protocols are necessary and crucial to optimize outcomes and patient satisfaction and reduce health care costs.
Methods:This work is based upon a literature search of published manuscripts (between 1996 and 2017) from Pubmed, Cochrane Central Register, and Google Scholar. It seeks to both synthesize and review our current scientific understanding of postcraniotomy pain and its part in neurosurgical ERAS protocols.
Results:Strategies to ameliorate craniotomy pain demand interventions during all phases of patient care: preoperative, intraoperative, and postoperative interventions. Pain management should begin in the perioperative period with risk assessment, patient education, and premedication. In the intraoperative period, modifications in anesthesia technique, choice of opioids, acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), regional techniques, dexmedetomidine, ketamine, lidocaine, corticosteroids, and interdisciplinary communication are all strategies to consider and possibly deploy. Opioids remain the mainstay for pain relief, but patient-controlled analgesia, NSAIDs, standardization of pain management, bio/behavioral interventions, modification of head dressings as well as patient-centric management are useful opportunities that potentially improve patient care.
Conclusions:Future research on mechanisms, predictors, treatments, and pain management pathways will help define the combinations of interventions that optimize pain outcomes.
Keywords: Analgesia, chronic pain, craniotomy, local anesthetics, neurosurgery
INTRODUCTION
Enhanced recovery after surgery (ERAS) protocols are designed to optimize outcomes, patient satisfaction, and reduce health care costs.[
Standardization of pain management is a key element of enhanced recovery protocols. Pain after craniotomy is a common occurrence[
Search strategy
This work is based on pertinent literature published from 1996, the date of a pivotal pilot study on craniotomy pain,[
Overview of eras approach to pain management
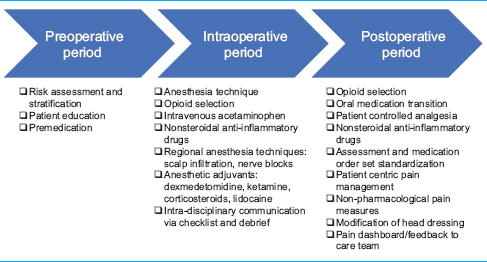
ERAS protocols divide the key components of perioperative care according to phase of care: preoperative, intraoperative, and postoperative interventions.[
Significance of poscraniotomy pain
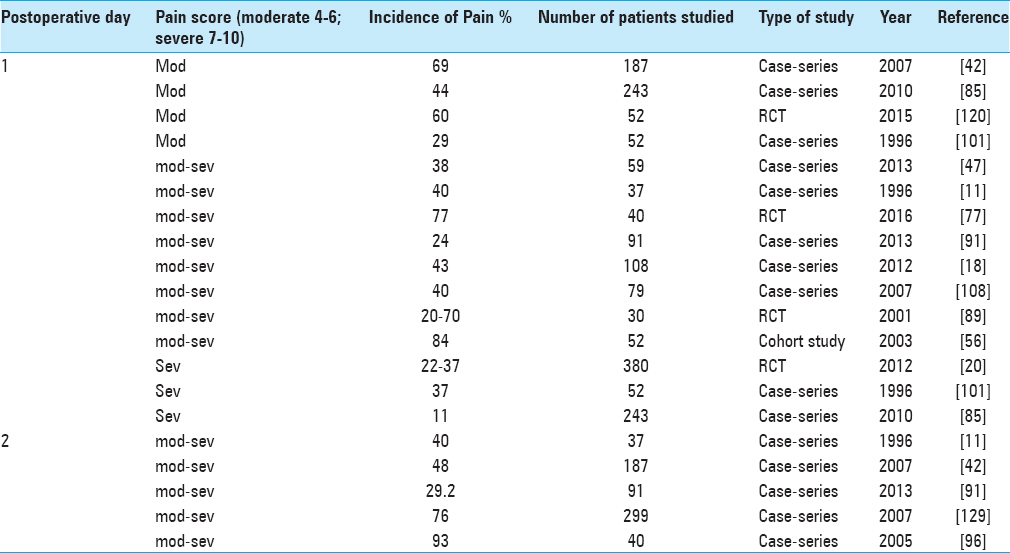
Pain after craniotomy is moderate to severe in up to 90% of patients within the first several days after the procedure.[
Origin of postcraniotomy pain
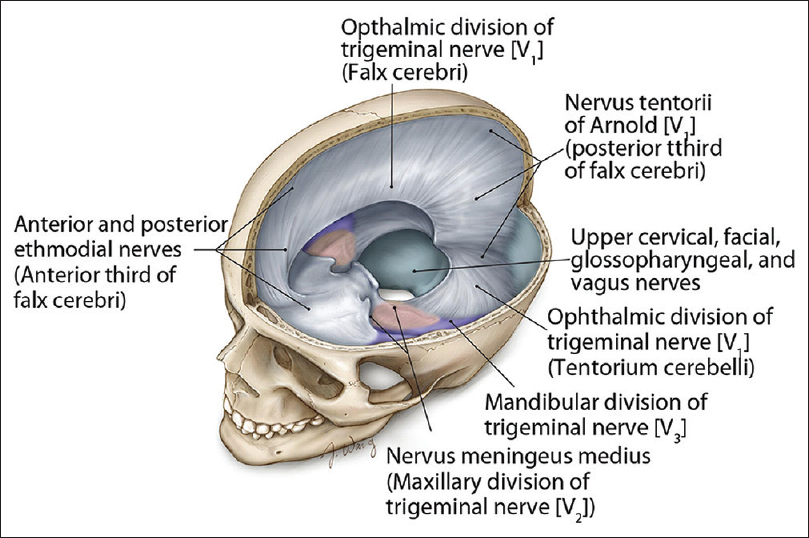
Innervation of the scalp is derived from cranial nerves and dorsal and ventral spinal rami. Branches of the ophthalmic division of the trigeminal nerve innervate the forehead. Branches of the mandibular and maxillary divisions of the trigeminal nerve innervate the skin of the temple. The greater occipital nerve innervates the posterior scalp and the lesser occipital nerve innervates the skin behind the ear. The dura is innervated by branches of the trigeminal nerve, ventral and dorsal rami of the cervical nerves, branches of the vagus, and hypoglossal nerves. The innervations for the various regions of the cranial dura mater are summarized in
Figure 1
Summary of the innervations for the various regions of the cranial dura mater. (Reprinted with permission[
Characteristics and time course
Acute postcraniotomy pain (ACP) is predominantly located to the area of incision, around occipital region and neck, and mainly involves pericranial muscle and soft tissues.[
Time-intensity curves showed that postcraniotomy pain is greatest in the first 48 h after surgery.[
Surgical procedures and their concomitant procedure specific pain syndromes are well recognized,[
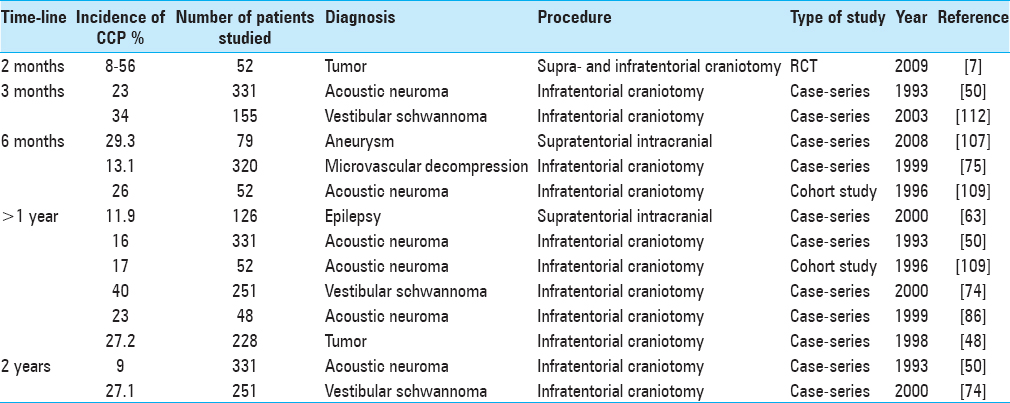
Ten studies that evaluated the incidence of CCP were identified and summarized in
Risk factors
Patient related risk factors
Independent predictors of severe postoperative pain after general anesthesia for various types of surgery are: younger age, female gender, level of preoperative pain, incision size, and type of surgery.[
Surgical procedure related risk factors
Craniotomy site may be a determinant for the type and severity of postoperative pain after neurosurgery.[
Risks factors for chronic postcraniotomy pain
CCP severity and incidence is greater after infratentorial procedures than supratentorial procedures [
Although no known surgical maneuver effectively prevents CCP, current recommended practice targets the restoration of muscular function, the rigid fixation of bone flaps, and cranioplasty in large craniotomies, the meticulous closing of the dura without tension, and the assiduous removal of blood and bone dust from intracranial contents.
The treatment of acute postcraniotomy pain has implications for long-term recovery: the severity of acute postsurgical pain predicts the incidence of chronic postsurgical pain after a number of surgical procedures.[
Perioperative interventions to improve pain experience after craniotomy
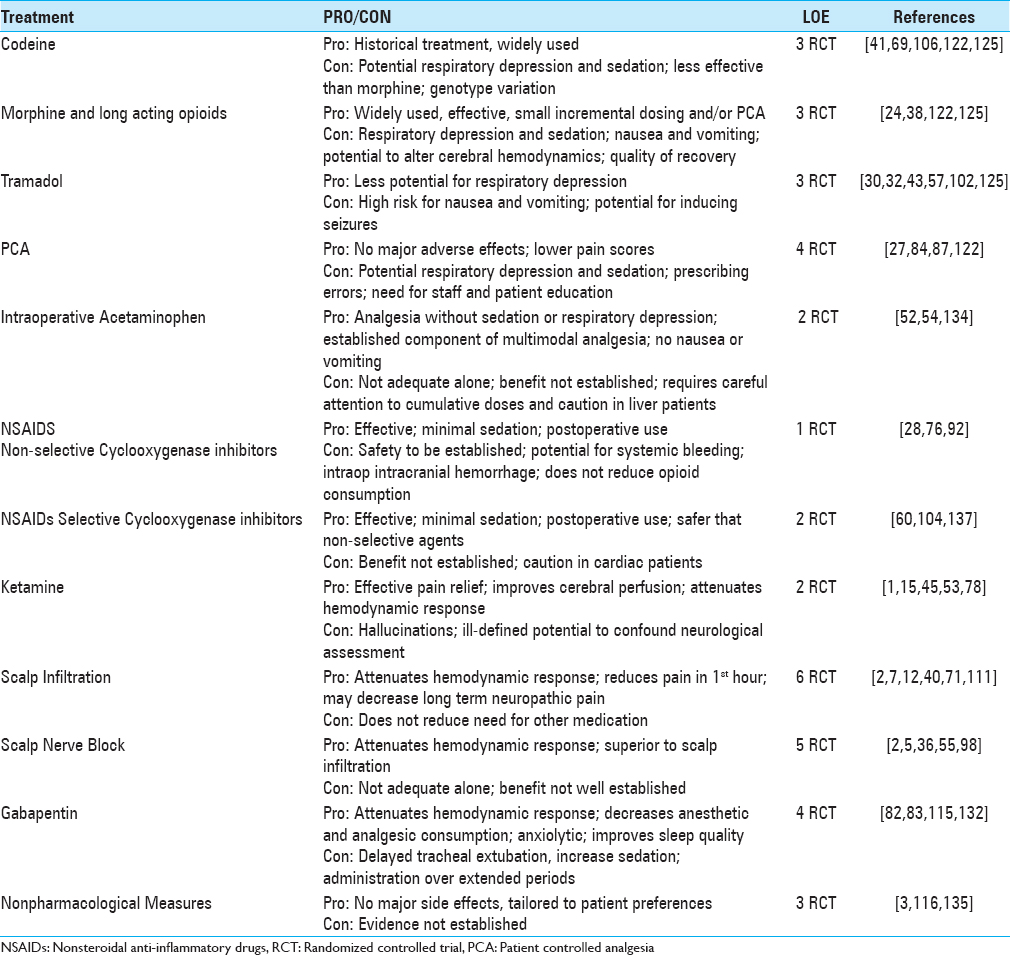
Strategies to ameliorate craniotomy pain demand interventions at all phases of patient care, these include: education, risk stratification, pain consultation, multimodal analgesia, and nonpharmacological and bio/behavioral interventions. Multimodal analgesia, the gold standard for management of perioperative pain, is an approach that combines treatments with additive or synergistic effects, reducing opioid consumption, controlling side effects, and improving overall outcomes. Tables
Preoperative interventions
Preoperative risk assessment
Identifying high-risk patients (anxiety, depression, and chronic pain) may improve pain management. The benefits include improved multidisciplinary communication about potential pain outcomes, risk adjusted therapeutic interventions and optimization or protocol variation based on risk assessment, and triggering pain consultation or behavioral cognitive intervention.[
Preoperative education
Surgical patients are concerned about pain and value content and communication about their pain experience.[
Preoperative medication
Enhanced recovery protocols endorse standardized preoperative administration of oral medications to mitigate pain. Preoperative gabapentin and acetaminophen administration are part of ERAS protocols for non-neurosurgical procedures.[
In patients undergoing craniotomy, preoperative gabapentin administration decreases anesthetic and analgesic consumption up to 48 h after surgery, but it also delays tracheal extubation and increased sedation postoperatively.[
Intraoperative interventions
Standardized anesthesia technique (inhalation versus intravenous anesthesia)
A small number of studies, not uniform in design, address anesthetic technique and postcraniotomy pain as well as other outcomes.[
Intraoperative opioid administration and transitional analgesia
The ultra-short-acting opioid remifentanil is widely used in neurosurgical anesthesia due to its favorable pharmacokinetic profile, but its use is debated in the context of improving postoperative pain experience. Remifentanil has a dose-dependent potential to amplify postoperative pain and induce pain sensitization.[
Intravenous acetaminophen
As clinical examination of the awake patient is the mainstay of complication surveillance after craniotomy, medications that provide analgesia without additive sedation are particularly valuable. The administration of acetaminophen is unlikely to cause significant additive sedation and likely to provide additive analgesia. Alone it is not potent enough to control pain after craniotomy[
NSAIDS
The intraoperative use of non-selective COX-1/COX-2 inhibitors for the patient undergoing craniotomy is questionable. Due to antiplatelet effects, preoperative use can be linked to intracranial hemorrhage in 1.1% of patients.[
Scalp infiltration
Infiltration of the scalp with solutions of local anesthetics is widely performed during neurosurgery. When solutions containing epinephrine are used this technique achieves local vasoconstriction and reduces scalp bleeding. Scalp infiltration also decreases the hemodynamic response to placement of head fixation devices and surgical incision.[
Scalp nerve block
Regional scalp block (SB) is an established technique that involves infiltration of local anesthetic to seven nerves on either side of the head, targeting the major sensory innervation of the scalp.[
The precise assessment of specific side effects and complications for the use of SB is precluded by the small sample sizes of available studies.[
Dexmedetomidine
Several studies support a role for intraoperative dexmedetomidine in mitigating postcraniotomy pain. Dexmedetomidine has an opioid-sparing effect[
Ketamine
Ketamine, a phencyclidine derivative with N-methyl-D-aspartate (NMDA) receptor antagonist properties,[
Corticosteroids
Corticosteroids, namely dexamethasone, are frequently administered perioperatively in patients undergoing craniotomy in order to mitigate cerebral edema and PONV. The absence of dexamethasone during craniotomy appears to increase postcraniotomy pain.[
Lidocaine infusion
Perioperative intravenous lidocaine infusion is a component of several enhanced recovery protocols for non-neurosurgical procedures. A review of RCTs revealed improvement in early postoperative pain in patient undergoing abdominal surgery.[
Pain management in surgical safety checklist and debrief
The surgical safety checklist includes opportunities to share information about pain risk factors and pain management plans. This can take place before skin incision where the anesthesia team can review patient specific concerns, and at debrief prior to leaving the operating room when the surgeon, the anesthesia professional, and the nurse review the key concerns for recovery and management of the patient.[
Postoperative interventions
Opioid administration
Opioids are the mainstay treatment for early postcraniotomy pain despite a wide array of side effects. The concerns about interference with early postoperative neurologic examination, respiratory depression, nausea, and over sedation are well founded. Small doses, careful titration, and monitoring are emphasized. Most centers administer opioids on an as-needed basis.[
Tramadol is less likely to cause respiratory depression compared to other opioids. Despite this potential advantage for neurosurgical patients, tramadol does share the negative side effects of other opioids namely nausea, vomiting, sedation, and drowsiness.[
Patient-controlled analgesia
Patient-controlled analgesia (PCA) is another option for postcraniotomy pain treatment. Limited studies show it to be subjectively better than nurse-administered analgesia.[
Postoperative NSAIDS
Postoperative administration of non-selective COX-1/COX-2 inhibitors, such as ketorolac, in the early postoperative period is an area of controversy.[
Consistent postoperative pain management
Part of ERAS protocols is standardization of postoperative pain orders, pain assessments, side effect appraisals, and early switch to oral medication. The goal is to provide consistent analgesia and minimize breakthrough pain.[
Patient-centric pain management
Patient-centric pain management may reduce the likelihood of over treatment with opioids. Prior studies showed that patients may consider pain tolerable and not desire treatment despite the intensity of pain.[
Nonpharmacological pain reduction techniques
Nonpharmacologic therapies for postsurgical pain include the application of heat and cold, massage therapy, aromatherapy, guided imagery, music therapy, biofeedback, hypnosis, and acupuncture. Live music therapy using patient preferred music has shown to decrease anxiety and stress, but not pain or analgesic requirements, after elective craniotomy.[
Modification of head dressings
Patients complain of discomfort related to the tightness of the circumferential head dressings used to reduce the risk of subgaleal fluid collection. Formal analysis or review of the type of dressing and its relationship to pain experience has not yet been performed. Skin necrosis is reported as a complication of a head dressing wrapped too tightly.[
Feedback to care team using pain dashboard
Dashboards can drive compliance with patient care protocols.[
SUMMARY AND CONCLUSIONS
The study of postcraniotomy pain is challenging because of several confounding variables. These include the use of different intraoperative anesthetics/opioids, lack of standardized postoperative pain management protocols, subjectivity of pain assessment techniques, and the patients’ neurological status.[
This current review outlines the options pertinent to the perioperative management of craniotomy pain. Information on perioperative pain management options is widely available from research studies, quality improvement trials, and enhanced recovery protocols for non-neurosurgical procedures. Examination of procedure specific foundation for each care management option reveals a paucity of randomized controlled and data driven studies,[
The potential benefits of standardized perioperative pain management pathways include simplification, decreased variation, and reduced possibility of error as well as improved outcomes. Creating a pathway that requires consensus between nurses, physicians, and allied professionals also provides an opportunity for the entire perioperative care team to review local pain management processes and the objective evidence supporting each care management intervention.
Pain management begins in the preoperative period with risk assessment, patient education, and administration of oral medications, when appropriate. While the modification of operative techniques might be useful in pain reduction, anesthetic management, and the use of diferent analgesic techniques, such as regional blocks, adjuvants, or alpha-2 adrenergic agonists, are potentially important areas of interest. Opioids are still the mainstay treatment for postcraniotomy pain, but several other interventions have the potential to improve outcomes. Multimodal analgesia, nonpharmacological techniques, standardized pain management protocols, and empowering the patient in the management of their pain are all possible avenues for success. Future research on mechanisms, predictors, treatments, and pain management pathways will help define the combinations of interventions that optimize pain outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal A, Sinha PK, Pandey CM, Gaur A, Pandey CK, Kaushik S. Effect of a subanesthetic dose of intravenous ketamine and/or local anesthetic infiltration on hemodynamic responses to skull-pin placement: A prospective, placebo-controlled, randomized, double-blind study. J Neurosurg Anesthesiol. 2001. 13: 189-94
2. Akcil EF, Dilmen OK, Vehid H, Ibısoglu LS, Tunali Y. Which one is more effective for analgesia in infratentorial craniotomy? The scalp block or local anesthetic infiltration. Clin Neurol Neurosurg. 2017. 154: 98-103
3. An L-X, Chen X, Ren X-J, Wu H-F. Electro-acupuncture decreases postoperative pain and improves recovery in patients undergoing a supratentorial craniotomy. Am J Chin Med. 2014. 42: 1099-109
4. Avidan MS, Maybrier HR, Abdallah A Ben, Jacobsohn E, Vlisides PE, Pryor KO. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: An international, multicentre, double-blind, randomised clinical trial. Lancet. 2017. 390: 230-
5. Bala I, Gupta B, Bhardwaj N, Ghai B, Khosla VK. Effect of scalp block on postoperative pain relief in craniotomy patients. Anaesth Intensive Care. 2006. 34: 224-7
6. Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000. 93: 48-54
7. Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg. 2009. 109: 240-4
8. Bekker A, Haile M, Kline R, Didehvar S, Babu R, Martiniuk F. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol. 2013. 25: 16-24
9. Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E. The Effect of Dexmedetomidine on Perioperative Hemodynamics in Patients Undergoing Craniotomy. Anesth Analg. 2008. 107: 1340-7
10. Benditz A, Greimel F, Auer P, Zeman F, Göttermann A, Grifka J. Can consistent benchmarking within a standardized pain management concept decrease postoperative pain after total hip arthroplasty? A prospective cohort study including 367 patients. J Pain Res. 2016. 9: 1205-13
11. Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: A pilot study in brain surgery. Neurosurgery. 1996. 38: 466-
12. Biswas BK, Bithal PK. Preincision 0.25% bupivacaine scalp infiltration and postcraniotomy pain: A randomized double-blind, placebo-controlled study. J Neurosurg Anesthesiol. 2003. 15: 234-9
13. Bloomfield EL, Schubert A, Secic M, Barnett G, Shutway F, Ebrahim ZY. The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg. 1998. 87: 579-82
14. Boostani R, Derakhshan S. Tramadol induced seizure: A 3-year study. Casp J Intern Med. 2012. 3: 484-7
15. Bourgoin A, Albanèse J, Wereszczynski N, Charbit M, Vialet R, Martin C. Safety of sedation with ketamine in severe head injury patients: Comparison with sufentanil. Crit Care Med. 2003. 31: 711-7
16. Burnand C, Sebastian J. Anaesthesia for awake craniotomy. Contin Educ Anaesth Crit Care Pain. 2014. 14: 6-11
17. Catalano PJ, Jacobowitz O, Post KD. Prevention of headache after retrosigmoid removal of acoustic tumors. Am J Otol. 1996. 17: 904-8
18. Ceylan A, Derbent A, Gokmen N, Anadolu O, Karaman S, Uyer M. Gender difference in early pain after craniotomy. J Neurol Sci Turk. 2012. 29: 248-57
19. Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005. 119: 1396-402
20. Citerio G, Pesenti A, Latini R, Masson S, Barlera S, Gaspari F. A multicentre, randomised, open-label, controlled trial evaluating equivalence of inhalational and intravenous anaesthesia during elective craniotomy. Eur J Anaesthesiol. 2012. 29: 371-9
21. Clark DJAccessed on 14 June 2017. Available: https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/painmedicine/16/9/10.1111/pme.12796/2/16-9-1666.pdf?Expires=1497560968&Signature=NxKLoxNeIKACBrEOxJPXULf6SHUSNvhP1q10KlylALp3pxNicxTS96EVo2f-o9lgkVNoGLSYEsYge8ci7j2pzFBNRlIKD7niz4ooAtd057.
22. Cohen-Gadol A, Kemp W, Tubbs Rs. The innervation of the scalp: A comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int. 2011. 2: 178-
23. Cohen NL. Retrosigmoid approach for acoustic tumor removal. Otolaryngol Clin North Am. 1992. 25: 295-310
24. Cold GE, Felding M. Even small doses of morphine might provoke luxury perfusion in the postoperative period after craniotomy. Neurosurgery. 1993. 32: 327-
25. Dangayach NS, Caridi J, Bederson J, Mayer SA. Enhanced Recovery After Neurosurgery: Paradigm Shift and Call to Arms. World Neurosurg. 2017. 100: 683-5
26. Dijk JFM, van Wijck AJM, Kappen TH, Peelen LM, Kalkman CJ, Schuurmans MJ. The Effect of a Preoperative Educational Film on Patients’ Postoperative Pain in Relation to their Request for Opioids. Pain Manag Nurs. 2015. 16: 137-45
27. Dilmen OK, Akcil EF, Tunali Y, Karabulut ES, Bahar M, Altindas F. Postoperative analgesia for supratentorial craniotomy. Clin Neurol Neurosurg. 2016. 146: 90-5
28. Dolmatova E V, Imaev AA, Lubnin AY. “Scheduled” dosing of lornoxicam provides analgesia superior to that provided by “on request” dosing following craniotomy. Eur J Anaesthesiol. 2009. 26: 633-7
29. Driscoll CL, Beatty CW. Pain after acoustic neuroma surgery. Otolaryngol Clin North Am. 1997. 30: 893-903
30. Evers AS, Maze M, Kharasch ED.editorsAnesthetic Pharmacology: Basic Principles and Clinical Practice. Cambridge University Press; 2011. p.
31. Felden L, Walter C, Harder S, Treede R-D, Kayser H, Drover D. Comparative clinical effects of hydromorphone and morphine: A meta-analysis. Br J Anaesth. 2011. 107: 319-28
32. Ferber J, Juniewicz H, Głogowska E, Wroński J, Abraszko R, Mierzwa J. Tramadol for postoperative analgesia in intracranial surgery. Its effect on ICP and CPP. Neurol Neurochir Pol. 2000. 34: 70-9
33. Finegold H, Stacey BR. Epidural blood patch to treat persistent headache after retromastoid craniectomy. Reg Anesth. 1996. 21: 602-3
34. Flexman AM, Ng JL, Gelb AW. Acute and chronic pain following craniotomy. Curr Opin Anaesthesiol. 2010. 23: 551-7
35. Garson L, Schwarzkopf R, Vakharia S, Alexander B, Stead S, Cannesson M. Implementation of a Total Joint Replacement-Focused Perioperative Surgical Home. Anesth Analg. 2014. 118: 1081-9
36. Gazoni FM, Pouratian N, Nemergut EC. Effect of ropivacaine skull block on perioperative outcomes in patients with supratentorial brain tumors and comparison with remifentanil: A pilot study. J Neurosurg. 2008. 109: 44-9
37. Ge D-J, Qi B, Tang G, Li J-Y. Intraoperative Dexmedetomidine Promotes Postoperative Analgesia and Recovery in Patients after Abdominal Colectomy: A CONSORT-Prospective, Randomized, Controlled Clinical Trial. Medicine (Baltimore). 2015. 94: e1727-
38. Gelb AW, Salevsky F, Chung F, Ringaert K, McTaggart-Cowan RMC, Wong T. Remifentanil with morphine transitional analgesia shortens neurological recovery compared to fentanyl for supratentorial craniotomy. Can J Anaesth. 2003. 50: 946-52
39. Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: A cut-off point analysis applying four different methods. Br J Anaesth. 2011. 107: 619-26
40. Geze S, Yilmaz AA, Tuzuner F. The effect of scalp block and local infiltration on the haemodynamic and stress response to skull-pin placement for craniotomy. Eur J Anaesthesiol. 2009. 26: 298-303
41. Goldsack C, Scuplak SM, Smith M. A double-blind comparison of codeine and morphine for postoperative analgesia following intracranial surgery. Anaesthesia. 1996. 51: 1029-32
42. Gottschalk A, Berkow LC, Stevens RD, Mirski M, Thompson RE, White ED. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg. 2007. 106: 210-6
43. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004. 43: 879-923
44. Guilfoyle MR, Helmy A, Duane D, Hutchinson PJA. Regional scalp block for postcraniotomy analgesia: A systematic review and meta-analysis. Anesth Analg. 2013. 116: 1093-102
45. Hagan KB, Bhavsar S, Raza SM, Arnold B, Arunkumar R, Dang A. Enhanced recovery after surgery for oncological craniotomies. J Clin Neurosci. 2016. 24: 10-6
46. Hansen MS, Brennum J, Moltke FB, Dahl JB. Pain treatment after craniotomy: Where is the (procedure-specific) evidence? A qualitative systematic review. Eur J Anaesthesiol. 2011. 28: 821-9
47. Hansen MS, Brennum J, Moltke FB, Dahl JB. Suboptimal pain treatment after craniotomy. Dan Med J. 2013. 60: A4569-
48. Hanson MB, Glasscock ME, Brandes JL, Jackson CG. Medical treatment of headache after suboccipital acoustic tumor removal. Laryngoscope. 1998. 108: 1111-4
49. Harkins SW, Price DD, Martelli M. Effects of age on pain perception: Thermonociception. J Gerontol. 1986. 41: 58-63
50. Harner SG, Beatty CW, Ebersold MJ. Headache after acoustic neuroma excision. Am J Otol. 1993. 14: 552-5
51. Harner SG, Beatty CW, Ebersold MJ. Impact of cranioplasty on headache after acoustic neuroma removal. Neurosurgery. 1995. 36: 1097-
52. Hassani E, Mahoori A, Sane S, Tolumehr A. Comparison the effects of paracetamol with sufentanil infusion on postoperative pain control after craniotomy in patients with brain tumor. Adv Biomed Res. 2015. 4: 64-
53. Himmelseher S, Durieux ME. Revising a Dogma: Ketamine for Patients with Neurological Injury?. Anesth Analg. 2005. 101: 524-34
54. Hoefnagel A, Lopez M, Smith M, Feng C, Nadler J. Intravenous Acetaminophen Administration in Patients Undergoing Craniotomy - A Retrospective Institutional Study. J Anesth Clin Res. 2015. 6: 1-4
55. Hwang J-Y, Bang J-S, Oh C-W, Joo J-D, Park S-J, Do S-H. Effect of scalp blocks with levobupivacaine on recovery profiles after craniotomy for aneurysm clipping: A randomized, double-blind, and controlled study. World Neurosurg. 2015. 83: 108-13
56. Irefin SA, Schubert A, Bloomfield EL, DeBoer GE, Mascha EJ, Ebrahim ZY. The effect of craniotomy location on postoperative pain and nausea. J Anesth. 2003. 17: 227-31
57. Jeffrey HM, Charlton P, Mellor DJ, Moss E, Vucevic M. Analgesia after intracranial surgery: A double-blind, prospective comparison of codeine and tramadol. Br J Anaesth. 1999. 83: 245-9
58. Jellish WS, Murdoch J, Leonetti JP. Perioperative management of complex skull base surgery: The anesthesiologist's point of view. Neurosurg Focus. 2002. 12: e5-
59. Jian M, Li X, Wang A, Zhang L, Han R, Gelb AW. Flurbiprofen and hypertension but not hydroxyethyl starch are associated with post-craniotomy intracranial haematoma requiring surgery. Br J Anaesth. 2014. 113: 832-9
60. Jones SJ, Cormack J, Murphy MA, Scott DA. Parecoxib for analgesia after craniotomy. Br J Anaesth. 2009. 102: 76-9
61. Kalkman CJ, Visser K, Moen J, Bonsel GJ, Grobbee DE, Moons KGM. Preoperative prediction of severe postoperative pain. Pain. 2003. 105: 415-23
62. Kastanias P, Denny K, Robinson S, Sabo K, Snaith K. What do adult surgical patients really want to know about pain and pain management?. Pain Manag Nurs. 2009. 10: 22-31
63. Kaur A, Selwa L, Fromes G, Ross DA. Persistent headache after supratentorial craniotomy. Neurosurgery. 2000. 47: 633-6
64. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006. 367: 1618-25
65. Kelly KP, Janssens MC, Ross J, Horn EH. Controversy of non-steroidal anti-inflammatory drugs and intracranial surgery: Et ne nos inducas in tentationem?. Br J Anaesth. 2011. 107: 302-5
66. Kemp WJ, Tubbs RS, Cohen-Gadol AA. The Innervation of the Cranial Dura Mater: Neurosurgical Case Correlates and a Review of the Literature. World Neurosurg. 2012. 78: 505-10
67. Klimek M, Ubben JFH, Ammann J, Borner U, Klein J, Verbrugge SJC. Pain in neurosurgically treated patients: A prospective observational study. J Neurosurg. 2006. 104: 350-9
68. Kol E, Alpar ŞE, Erdoǧan A. Preoperative Education and Use of Analgesic Before Onset of Pain Routinely for Post-thoracotomy Pain Control Can Reduce Pain Effect and Total Amount of Analgesics Administered Postoperatively. Pain Manag Nurs. 2014. 15: 331-9
69. Kotak D, Cheserem B, Solth A. A survey of post-craniotomy analgesia in British neurosurgical centres: Time for perceptions and prescribing to change?. Br J Neurosurg. 2009. 23: 538-42
70. Kranke P, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane database Syst Rev. 2015. p.
71. Law-Koune JD, Szekely B, Fermanian C, Peuch C, Liu N, Fischler M. Scalp infiltration with bupivacaine plus epinephrine or plain ropivacaine reduces postoperative pain after supratentorial craniotomy. J Neurosurg Anesthesiol. 2005. 17: 139-43
72. Leslie K, Troedel S, Irwin K, Pearce F, Ugoni A, Gillies R. Quality of recovery from anesthesia in neurosurgical patients. Anesthesiology. 2003. 99: 1158-65
73. Leung JL, Sands LP, Rico M, Petersen KL, Rowbotham MC, Dahl JB. Pilot clinical trial of gabapentin to decrease postoperative delirium in older surgical patients. Neurology. 2006. 67: 1-3
74. Levo H, Pyykkö I, Blomstedt G. Postoperative headache after surgery for vestibular schwannoma. Ann Otol Rhinol Laryngol. 2000. 109: 853-8
75. Lovely TJ, Lowry DW, Jannetta PJ. Functional outcome and the effect of cranioplasty after retromastoid craniectomy for microvascular decompression. Surg Neurol. 1999. 51: 191-7
76. Magni G, La Rosa I, Melillo G, Abeni D, Hernandez H, Rosa G. Intracranial hemorrhage requiring surgery in neurosurgical patients given ketorolac: A case-control study within a cohort (2001-2010). Anesth Analg. 2013. 116: 443-7
77. Markovic-Bozic J, Karpe B, Potocnik I, Jerin A, Vranic A, Novak-Jankovic V. Effect of propofol and sevoflurane on the inflammatory response of patients undergoing craniotomy. BMC Anesthesiol. 2016. 16: 18-
78. Mayberg TS, Lam AM, Matta BF, Domino KB, Winn HR. Ketamine does not increase cerebral blood flow velocity or intracranial pressure during isoflurane/nitrous oxide anesthesia in patients undergoing craniotomy. Anesth Analg. 1995. 81: 84-9
79. McNicholas E, Bilotta F, Titi L, Chandler J, Rosa G, Koht A. Transient facial nerve palsy after auriculotemporal nerve block in awake craniotomy patients. A A Case Rep. 2014. 2: 40-3
80. Melzack R. From the gate to the neuromatrix. Pain. 1999. p. S121-6
81. Michtalik HJ, Carolan HT, Haut ER, Lau BD, Streiff MB, Finkelstein J. Use of provider-level dashboards and pay-for-performance in venous thromboembolism prophylaxis. J Hosp Med. 2015. 10: 172-8
82. Misra S, Koshy T, Unnikrishnan KP, Suneel PR, Chatterjee N. Gabapentin premedication decreases the hemodynamic response to skull pin insertion in patients undergoing craniotomy. J Neurosurg Anesthesiol. 2011. 23: 110-7
83. Misra S, Parthasarathi G, Vilanilam GC. The effect of gabapentin premedication on postoperative nausea, vomiting, and pain in patients on preoperative dexamethasone undergoing craniotomy for intracranial tumors. J Neurosurg Anesthesiol. 2013. 25: 386-91
84. Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE. Efficacy of intravenous patient-controlled analgesia after supratentorial intracranial surgery: A prospective randomized controlled trial. Clinical article. J Neurosurg. 2009. 111: 343-50
85. Mordhorst C, Latz B, Kerz T, Wisser G, Schmidt A, Schneider A. Prospective assessment of postoperative pain after craniotomy. J Neurosurg Anesthesiol. 2010. 22: 202-6
86. Mosek AC, Dodick DW, Ebersold MJ, Swanson JW. Headache After Resection of Acoustic Neuroma. Headache J Head Face Pain. 1999. 39: 89-94
87. Na H-S, An S-B, Park H-P, Lim Y-J, Hwang J-W, Jeon Y-T. Intravenous patient-controlled analgesia to manage the postoperative pain in patients undergoing craniotomy. Korean J Anesthesiol. 2011. 60: 30-5
88. Hughey AB, Lesniak MS, Ansari SA, Roth S. What will anesthesiologists be anesthetizing. Trends in neurosurgical procedure usage?. Anesthesia & Analgesia. 2010. 110: 1686-97
89. Nguyen A, Girard F, Boudreault D, Fugère F, Ruel M, Moumdjian R. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. 2001. 93: 1272-6
90. Nielsen R V, Siegel H, Fomsgaard JS, Andersen JDH, Martusevicius R, Mathiesen O. Preoperative dexamethasone reduces acute but not sustained pain after lumbar disk surgery: A randomized, blinded, placebo-controlled trial. Pain. 2015. 156: 2538-44
91. Oliveira Ribeiro M do C, Pereira CU, Sallum AM, Martins-Filho PRS, Desantana JM, da Silva Nunes M. Immediate post-craniotomy headache. Cephalalgia. 2013. 33: 897-905
92. Palmer JD, Sparrow OC, Iannotti F. Postoperative hematoma: A 5-year survey and identification of avoidable risk factors. Neurosurgery. 1994. 35: 1061-
93. Papangelou A, Radzik BR, Smith T, Gottschalk A. A review of scalp blockade for cranial surgery. J Clin Anesth. 2013. 25: 150-9
94. Peng K, Jin X, Liu S, Ji F. Effect of Intraoperative Dexmedetomidine on Post-Craniotomy Pain. Clin Ther. 2015. 37: 1114-21.e1
95. Peng Y, Zhang W, Kass IS, Han R. Lidocaine Reduces Acute Postoperative Pain After Supratentorial Tumor Surgery in the PACU. J Neurosurg Anesthesiol. 2016. 28: 309-15
96. Peón AU, Diccini S. [Postoperative pain in craniotomy]. Rev Lat Am Enfermagem. 2005. 13: 489-95
97. Perks A, Chakravarti S, Manninen P. Preoperative Anxiety in Neurosurgical Patients. J Neurosurg Anesthesiol. 2009. 21: 127-30
98. Pinosky ML, Fishman RL, Reeves ST, Harvey SC, Patel S, Palesch Y. The effect of bupivacaine skull block on the hemodynamic response to craniotomy. Anesth Analg. 1996. 83: 1256-61
99. Prabhakar H, Singh GP, Mahajan C, Kapoor I, Kalaivani M, Anand V, Prabhakar H.editors. Intravenous versus inhalational techniques for rapid emergence from anaesthesia in patients undergoing brain tumour surgery. Cochrane Database of Systematic Reviews. UK: John Wiley and Sons, Ltd; 2016. p.
100. Prabhakar S, Nanavati AJ. Enhanced Recovery After Surgery: If You Are Not Implementing it, Why Not? Nutr. Issues Gastroenterol. 2016. p.
101. Quiney N, Cooper R, Stoneham M, Walters F. Pain after craniotomy. A time for reappraisal?. Br J Neurosurg. 1996. 10: 295-9
102. Rahimi SY, Alleyne CH, Vernier E, Witcher MR, Vender JR, Alleyne CH. Postoperative pain management with tramadol after craniotomy: Evaluation and cost analysis Clinical article. J Neurosurg. 2010. 112: 268-72
103. Rajan S, Hutcherson MT, Sessler DI, Kurz A, Yang D, Ghobrial M. The effects of dexmedetomidine and remifentanil on hemodynamic stability and analgesic requirement after craniotomy: A randomized controlled trial. J Neurosurg Anesthesiol. 2016. 28: 282-90
104. Rao GSU, Gelb AW. To use or not to use: The dilemma of NSAIDs and craniotomy. Eur J Anaesthesiol. 2009. 26: 625-6
105. Rimaaja T, Haanpaa M, Blomstedt G, Farkkila M, Haanpää M, Blomstedt G. Headaches after acoustic neuroma surgery. Cephalgia. 2007. 27: 1128-35
106. Roberts GC. Post-craniotomy analgesia: Current practices in British neurosurgical centres-a survey of post-craniotomy analgesic practices. Eur J Anaesthesiol. 2005. 22: 328-32
107. Rocha-Filho PAS, Gherpelli JLD, de Siqueira JTT, Rabello GD. Post-craniotomy headache: Characteristics, behaviour and effect on quality of life in patients operated for treatment of supratentorial intracranial aneurysms. Cephalalgia. 2008. 28: 41-8
108. Rocha-Filho P, Gherpelli J, de Siqueira J, Rabello G. Post-craniotomy headache: Characteristics, behaviour and effect on quality of life in patients operated for treatment of supratentorial intracranial aneurysms. Cephalalgia. 2008. 28: 41-8
109. Ruckenstein MJ, Harris JP, Cueva RA, Prioleau G, Alksne J. Pain subsequent to resection of acoustic neuromas via suboccipital and translabyrinthine approaches. Am J Otol. 1996. 17: 620-4
110. Samona J, Cook C, Krupa K, Swatsell K, Jackson A, Dukes C. Effect of Intraoperative Dexamethasone on Pain Scores and Narcotic Consumption in Patients Undergoing Total Knee Arthroplasty. Orthop Surg. 2017. 9: 110-4
111. Saringcarinkul A, Boonsri S. Effect of scalp infiltration on postoperative pain relief in elective supratentorial craniotomy with 0.5% bupivacaine with adrenaline 1:400,000. J Med Assoc Thai. 2008. 91: 1518-23
112. Schaller B, Baumann A. Headache after removal of vestibular schwannoma via the retrosigmoid approach: A long-term follow-up-study. Otolaryngol Head Neck Surg. 2003. 128: 387-95
113. Schessel DA, Nedzelski JM, Rowed D, Feghali JG. Pain after surgery for acoustic neuroma. Otolaryngol Head Neck Surg. 1992. 107: 424-9
114. Schessel DA, Rowed DW, Nedzelski JM, Feghali JG. Postoperative pain following excision of acoustic neuroma by the suboccipital approach: Observations on possible cause and potential amelioration. Am J Otol. 1993. 14: 491-4
115. Shimony N, Amit U, Minz B, Grossman R, Dany MA, Gonen L. Perioperative pregabalin for reducing pain, analgesic consumption, and anxiety and enhancing sleep quality in elective neurosurgical patients: A prospective, randomized, double-blind, and controlled clinical study. J Neurosurg. 2016. 125: 1513-22
116. Shin YS, Lim NY, Yun S-C, Park KO. A randomised controlled trial of the effects of cryotherapy on pain, eyelid oedema and facial ecchymosis after craniotomy. J Clin Nurs. 2009. 18: 3029-36
117. Sinatra R. Causes and Consequences of Inadequate Management of Acute Pain. Pain Med. 2010. 11: 1859-71
118. Singhal A, Bray PW, Bernstein M. Scalp ulceration from a circumferential head dressing after craniotomy: Case report of an uncommon complication due to human error. Can J Plast Surg. 2004. 12: 210-2
119. Song J, Ji Q, Sun Q, Gao T, Liu K, Li L. The Opioid-sparing Effect of Intraoperative Dexmedetomidine Infusion After Craniotomy. J Neurosurg Anesthesiol. 2016. 28: 14-20
120. Song J, Li L, Yu P, Gao T, Liu K. Preemptive scalp infiltration with 0.5% ropivacaine and 1% lidocaine reduces postoperative pain after craniotomy. Acta Neurochir (Wien). 2015. 157: 993-8
121. Soumekh B, Levine SC, Haines SJ, Wulf JA. Retrospective study of postcraniotomy headaches in suboccipital approach: Diagnosis and management. Am J Otol. 1996. 17: 617-9
122. Stoneham MD, Cooper R, Quiney NF, Walters FJ. Pain following craniotomy: A preliminary study comparing PCA morphine with intramuscular codeine phosphate. Anaesthesia. 1996. 51: 1176-8
123. Stoneham MD, Walters FJM. Postoperative analgesia for craniotomy patients - current attitudes among neuroanaesthetists. Eur J Anaesthesiol. 1995. 12: 571-5
124. Stricker PA, Kraemer FW, Ganesh A. Severe remifentanil-induced acute opioid tolerance following awake craniotomy in an adolescent. J Clin Anesth. 2009. 21: 124-6
125. Sudheer PS, Logan SW, Terblanche C, Ateleanu B, Hall JE. Comparison of the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy. Anaesthesia. 2007. 62: 555-60
126. Taenzer AH, Clark C, Curry CS. Gender affects report of pain and function after arthroscopic anterior cruciate ligament reconstruction. Anesthesiology. 2000. 93: 670-5
127. Talke PO, Gelb AW. Postcraniotomy pain remains a real headache!. Eur J Anaesthesiol. 2005. 22: 325-7
128. Tan M, Law LS-C, Gan TJ. Optimizing pain management to facilitate Enhanced Recovery After Surgery pathways. Can J Anaesth. 2015. 62: 203-18
129. Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: A retrospective study. Can J Anaesth. 2007. 54: 544-8
130. Titsworth WL, Abram J, Guin P, Herman MA, West J, Davis NW. A prospective time-series quality improvement trial of a standardized analgesia protocol to reduce postoperative pain among neurosurgery patients. J Neurosurg. 2016. 125: 1523-32
131. Tucker MA, Andrew MF, Ogle SJ, Davison JG. Age-associated change in pain threshold measured by transcutaneous neuronal electrical stimulation. Age Ageing. 1989. 18: 241-6
132. Tuere H, Sayin M, Karlikaya G, Bingol CA, Aykac B, Tuere U. The Analgesic Effect of Gabapentin as a Prophylactic Anticonvulsant Drug on Postcraniotomy Pain: A Prospective Randomized Study. Anesth Analg. 2009. 109: 1625-31
133. Last accessed on 2017 Sep 01. Available: http://neurosurgery.ucla.edu/Workfiles/Site-Neurosurgery/clinical-quality-program/2015-Neurosurgery-Quality-Report2.pdf.
134. Verchère E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol. 2002. 14: 96-101
135. Walworth D, Rumana CS, Nguyen J, Jarred J. Effects of live music therapy sessions on quality of life indicators, medications administered and hospital length of stay for patients undergoing elective surgical procedures for brain. J Music Ther. 2008. 45: 349-59
136. Last accessed on 2017 Sep 01. Available: http://apps.who.int/iris/bitstream/10665/44185/1/9789241598552_eng.pdf.
137. Williams DL, Pemberton E, Leslie K. Effect of intravenous parecoxib on post-craniotomy pain. Br J Anaesth. 2011. 107: 398-403
138. Yu EHY, Tran DHD, Lam SW, Irwin MG. Remifentanil tolerance and hyperalgesia: Short-term gain, long-term pain?. Anaesthesia. 2016. 71: 1347-62