- Department of Medicine, University of Hawai’i at Mānoa, John A. Burns School of Medicine, Honolulu, Hawaii, USA
- East-West Center, Brain Research, Innovation and Translation Lab, Honolulu, Hawaii, USA
- Hawai’i Pacific Neuroscience, Brain Research, Innovation and Translation Lab, Honolulu, Hawaii, USA
- Department of Neurological Surgery, University of California, Davis School of Medicine, Sacramento, California, USA
Correspondence Address:
Arash Ghaffari-Rafi, Department of Neurological Surgery, School of Medicine, University of California, Davis 4860 Y Street Suite 3740 Sacramento, CA, 95817, USA.
DOI:10.25259/SNI_190_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kyung Moo Kim1,2, Rachel Jane Lew1, Tate Justin Higashihara1, Shaina Yamashita1, Michelle Pang, Michelle Stafford1, Connor Goo1, Kimberly Bergenholtz Teehera1, Kayti Luu1, Richard Ho1, Enrique Carrazana1, Jason Viereck1,3, Kore Kai Liow1,3, Arash Ghaffari-Rafi1,4. Differences in tumor size, clinical, demographic, and socioeconomic profiles of central nervous system tumors among a racially diverse cohort: A retrospective case–control study. 13-Dec-2024;15:459
How to cite this URL: Kyung Moo Kim1,2, Rachel Jane Lew1, Tate Justin Higashihara1, Shaina Yamashita1, Michelle Pang, Michelle Stafford1, Connor Goo1, Kimberly Bergenholtz Teehera1, Kayti Luu1, Richard Ho1, Enrique Carrazana1, Jason Viereck1,3, Kore Kai Liow1,3, Arash Ghaffari-Rafi1,4. Differences in tumor size, clinical, demographic, and socioeconomic profiles of central nervous system tumors among a racially diverse cohort: A retrospective case–control study. 13-Dec-2024;15:459. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13290
Abstract
Background: One avenue to improve outcomes among brain tumor patients involves the mitigation of healthcare disparities. Investigating clinical differences among brain tumors across socioeconomic and demographic strata, such can aid in healthcare disparity identification and, by extension, outcome improvement.
Methods: Utilizing a racially diverse population from Hawaii, 323 cases of brain tumors (meningiomas, gliomas, schwannomas, pituitary adenomas, and metastases) were matched by age, sex, and race to 651 controls to investigate the associations between tumor type and various demographic, socioeconomic, and medical comorbidities. Tumor size at the time of diagnosis was also compared across demographic groups.
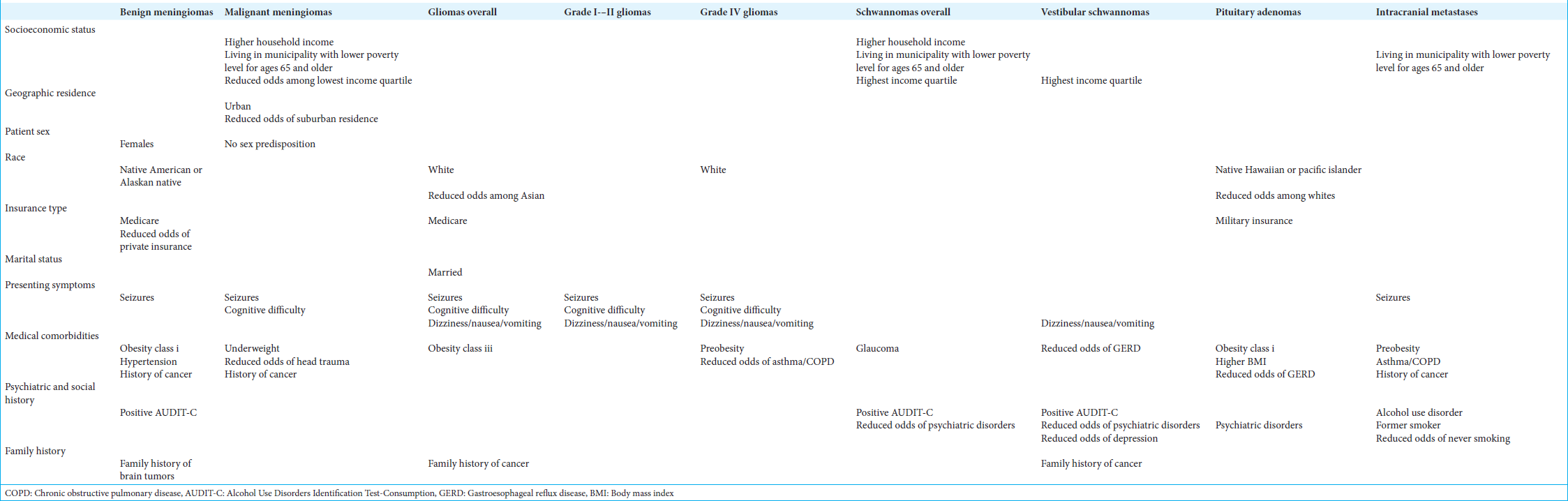
Results: At the time of diagnosis for benign meningiomas, Native Hawaiians and Pacific Islanders (NHPI; P P = 0.04) and Asians (P = 0.02), while for vestibular schwannomas, NHPI had larger tumor sizes compared to Asians (P P P P P
Conclusion: Brain tumors exhibit unique sociodemographic disparities and clinical comorbidities, which may have implications for diagnosis, treatment, and healthcare policy.
Keywords: Central nervous system, Disparities, Risk factors, Socioeconomic, Tumors
INTRODUCTION
Brain tumors impose a significant morbidity and mortality burden globally.[
MATERIALS AND METHODS
Design and setting
The electronic medical records of a neuroscience clinic in Honolulu, Hawaii (i.e., Hawaii Pacific Neuroscience) were retrospectively searched from January 1, 2009, to January 1, 2021. The following International Classification of Diseases 9th or 10th editions and Clinical Modification codes (ICD-9-CM or ICD-10-CM) for patients with benign intracranial tumors were used for 2015-2021: ICD-9-CM (225.0, 225.1, 225.2, 225.3, 225.4, 225.8, and 225.9) for 2009–2014, and ICD-10-CM (D32.0, D32.1, D32.9, D33.0, D33.1, D33.2, D33.3, D33.4, D33.7, D33.9, V12.41, and Z86.011). For malignant and miscellaneous intracranial tumors, the respective codes were applied: ICD-9-CM (191.0, 191.1, 191.2, 191.3, 191.4, 191.5, 191.6, 191.7, 191.8, 191.9, 192.0, 192.1, 192.2, 192.8, and 192.9) for 2009-2014 and ICD-10-CM (D42.0, D42.1, D42.9, V10.85, and Z85.841) for 2015– 2021. The Institutional Review Board approval was obtained before the study from the University of Hawai‘i Office of Research Compliance (protocol number: 2020-01010).
Predictor and outcome variables
For cases, the data for the following variables were collected: age at diagnosis, sex, presenting symptom, history of head trauma, history of stroke, presence of gait disturbances, seizures, cognitive difficulties, dizziness, nausea or vomiting (DNV), sleep disturbances, and self-identified race (White, Black, Hispanic/Latino, Asian, Native Hawaiian or Pacific Islander [NHPI], and Native American or Alaskan Natives [NAAN]). Tumor type and dimensions were attained from pathology and imaging reports. Tumor volume and area were calculated using the established formula for a spheroid:
V = volume, r = radius (half the diameter) along the longest dimension of the tumor along the axial (r1), coronal (r2), and sagittal (r3) planes.
A = area, r = radius (half the diameter) along the longest dimension of the tumor along either the axial, coronal, or sagittal plane.
The insurance and zone improvement plan code of the patient’s residence was collected as a proxy measure for median household income, in addition to the percentage or residence in a municipality below the poverty level (for all ages, 18–64 years, and 65 years and over). Such data were acquired from the United States Census Bureau, 2015–2019 American Community Survey 5-Year Estimates (http://www. census.gov). Insurance was classified as Medicare, Medicaid, private insurance, or military insurance, consistent with the criteria of the Agency of Health-care Research and Quality (Rockville, MD) for the Health-care Cost and Utilization Project (http://www.hcup-us.ahrq.gov).
The presence of the following cardiovascular risk factors was collected: type II diabetes mellitus, hypertension, atrial fibrillation/flutter, congestive heart failure (CHF), coronary artery disease or previous myocardial infarction, prosthetic valve replacement, and peripheral vascular disease. Associations between intracranial tumors and the following were also explored: autoimmune pathology, thyroid disorders, glaucoma, body mass index (BMI), obstructive sleep apnea, asthma or chronic obstructive pulmonary disease (COPD), and gastrointestinal diseases.
Social history elements collected included marital status and family histories of intracranial tumors, neurological disorders, stroke, and cancer. Self-reported smoking status (current, former, and never) was also collected. The smoking classification was based on the United States Centers for Disease Control and Prevention (CDC), National Health Interview Survey, and Adult Tobacco Use (https://www.cdc.gov/nchs/surveys.htm).
The collection of psychiatric risk factors included a history of depression and the extent of alcohol use. Depression was measured by the Patient Health Questionnaire-2 (PHQ-2) and Patient Health Questionnaire-9 (PHQ-9). The PHQ-2 and PHQ-9 are validated two-question and nine-question modules that detect and assess depression.[
Controls
Two to four controls were collected per case (n = 323) to maximize statistical power.[
Statistical analysis
The normality of data was assessed through quantile-quantile plots and histograms to determine parametric or non-parametric analysis. For categorical variables, either Pearson’s Chi-squared test or Fisher’s exact test of independence was chosen, while for non-parametric continuous variables, the independent Wilcoxon rank-sum test was used.[
RESULTS
All tumors
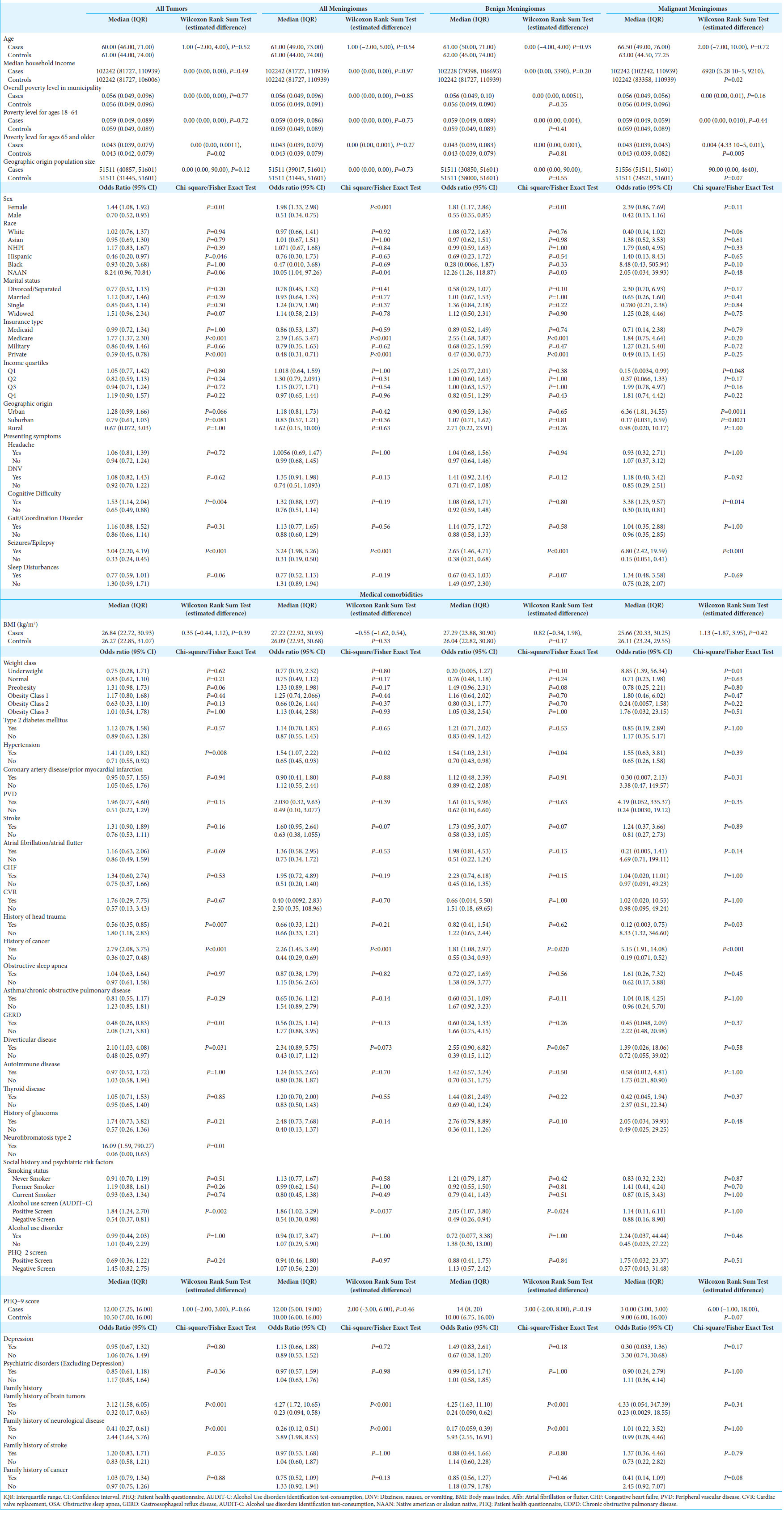
Cases of all intracranial tumors (n = 323) were compared to unmatched controls (n = 1292) [
Meningiomas
Of the analyzed meningiomas (n = 159), 81.1% were benign (n = 129) and 18.9% were malignant (n = 30) [
Socioeconomic variables
For benign meningiomas, patients had a 2.55 fold increased odds of having Medicare (95% CI: 1.68, 3.87; P < 0.001) and 0.47 fold decreased odds of having private insurance (95% CI: 0.30, 0.73; P < 0.001). Among malignant meningiomas, patients were at reduced odds of being from the first quartile (0.15, 95% CI: 0.0034, 0.99; P = 0.048). Meanwhile, geographically, malignant meningioma patients were at 6.36 fold greater odds of being from an urban location (95% CI: 1.81, 34.55; P = 0.001) and reduced odds of living in a suburban region (0.17, 95% CI: 0.031, 0.59; P = 0.002).
Presenting symptoms
For both benign (2.54, 95% CI: 1.47, 4.71; P < 0.001) and malignant meningiomas (6.80, 95% CI: 2.42, 19.59; P < 0.001), seizures were the most likely presentation; however, malignant meningioma patients were also more likely to present with cognitive difficulties (3.38, 95% CI: 1.23, 9.57; P = 0.014).
Medical comorbidities
In general, meningioma patients were found to have 1.86 times greater odds of a positive alcohol use screen (95% CI: 1.02, 3.29; P = 0.04). Benign meningiomas were specifically found to have increased odds of hypertension (1.54, 95% CI: 1.03, 2.31; P = 0.04), personal history of prior neoplasm (95% CI: 1.08, 2.97; P = 0.02), and family history of brain tumors (4.25, 95% CI: 1.63, 11.10; P < 0.001). Moreover, malignant meningioma patients not only had an increased odds of a history of prior neoplasm (5.15, 95% CI: 1.91, 14.08; P < 0.001) but also an 8.33 fold greater odds of head trauma history (95% CI: 1.32, 346.60; P = 0.03).
Multivariable analysis
Multivariable regression modeling was conducted to determine the best predictors of meningioma diagnosis. For benign meningiomas, variables that significantly increased the odds of diagnosis included: presentation with DNV (2.52, 95% CI: 1.25, 5.08; P = 0.01) or seizures (4.36, 95% CI: 1.78, 10.65; P = 0.001), presence of obesity class I (2.87, 95% CI: 1.08, 7.67; P = 0.04), CHF (6.64, 95% CI: 1.39, 31.73; P = 0.02), glaucomatous disease (9.80, 95% CI: 1.88, 51.06; P = 0.007), positive alcohol use screen (5.65, 95% CI: 2.38, 13.39; p < 0.001), history of stroke (3.05, 95% CI: 1.31, 7.08; P = 0.009) or neoplasm (2.26, 95% CI: 1.06, 4.81; P = 0.035), and family history of brain tumors (9.27, 95% CI: 1.84, 46.61; P = 0.007). In a best-fit model for malignant meningiomas, a presentation with seizures (8.25, 95% CI: 2.49, 27.33; P < 0.001) and a history of neoplasm (3.94, 95% CI: 1.12, 13.86; P = 0.03) were the strongest predictors of diagnosis.
Tumor size
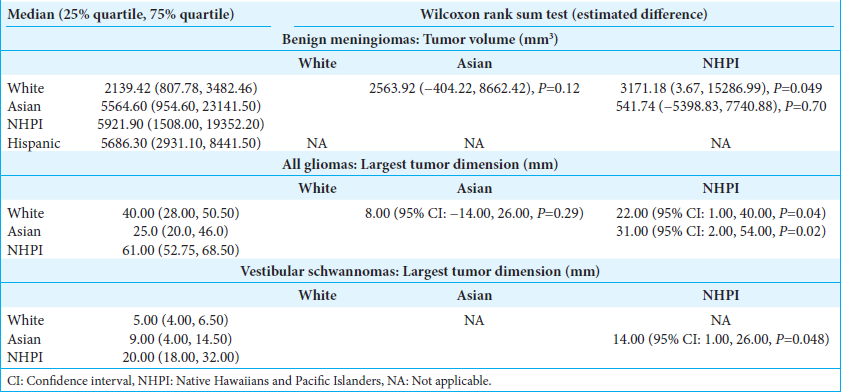
When tumor size was examined using three-dimensional and two-dimensional volumes, NHPI (3171.18 mm3, 95% CI: 3.67, 15286.99; P = 0.049) and Asian (219.00 mm3, 95% CI: 12.00, 668.00; P = 0.033) patients were found to have larger benign meningioma volumes at the time of diagnosis compared to Whites [
Gliomas
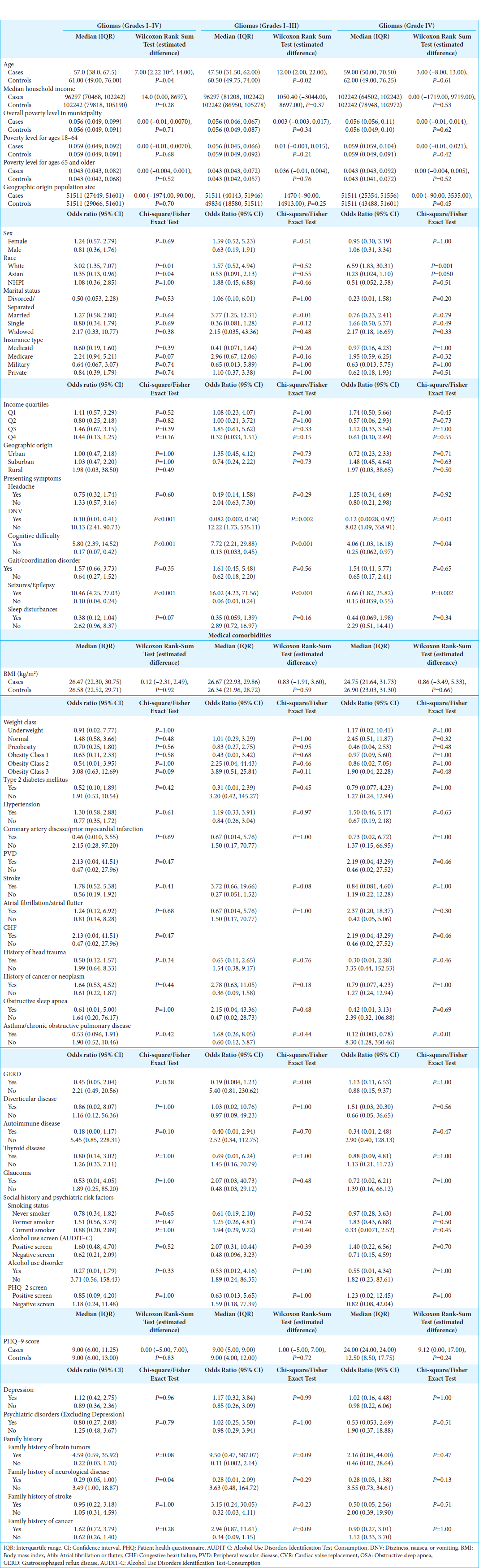
Of the 39 gliomas comprising the cohort, 51.3% were WHO Grades I–III gliomas (non-glioblastoma multiforme [GBM], n = 20) [
Seizures (non-GBM, OR: 16.8, 95% CI: 4.88–57.8, P < 0.001; GBM, OR: 6.86, 95% CI: 2.14–22.0, P = 0.001) and cognitive difficulties (non-GBM, OR: 7.94, 95% CI: 2.54–24.9, P < 0.001; GBM, OR: 4.14, 95% CI: 1.25–13.7, P = 0.020) were the most common presenting symptoms for all gliomas. Meanwhile, glioma patients had significantly reduced odds of presenting with DNV (non-GBM, 0.08, 95% CI: 0.01–0.64, P = 0.02; GBM, 0.12, 95% CI: 0.02–0.99; P = 0.049) or sleep disturbances (0.38, 95% CI: 0.15, 0.99; P = 0.048).
Glioma patients were also found to have increased odds of class III obesity (OR: 49.68, 95% CI: 1.59, 1550; P = 0.03), as well as a family history of cancer (OR: 22.6, 95% CI: 2.40, 213; P = 0.006). In multivariable analysis, the best predictor of glioma diagnosis was cognitive difficulty (non-GBM, OR: 13300, 95% CI: 5.98– 2.94 × 107, P = 0.02; GBM, OR: 61.7, 95% CI: 2.31–1650, P = 0.01).
Tumor size
NHPI patients had significantly larger tumor dimensions compared to White (22.00 mm, 95% CI: 1.00, 40.00, P = 0.04) and Asian (31.00 mm, 95% CI: 2.00, 54.00, P = 0.02) patients [
Schwannomas
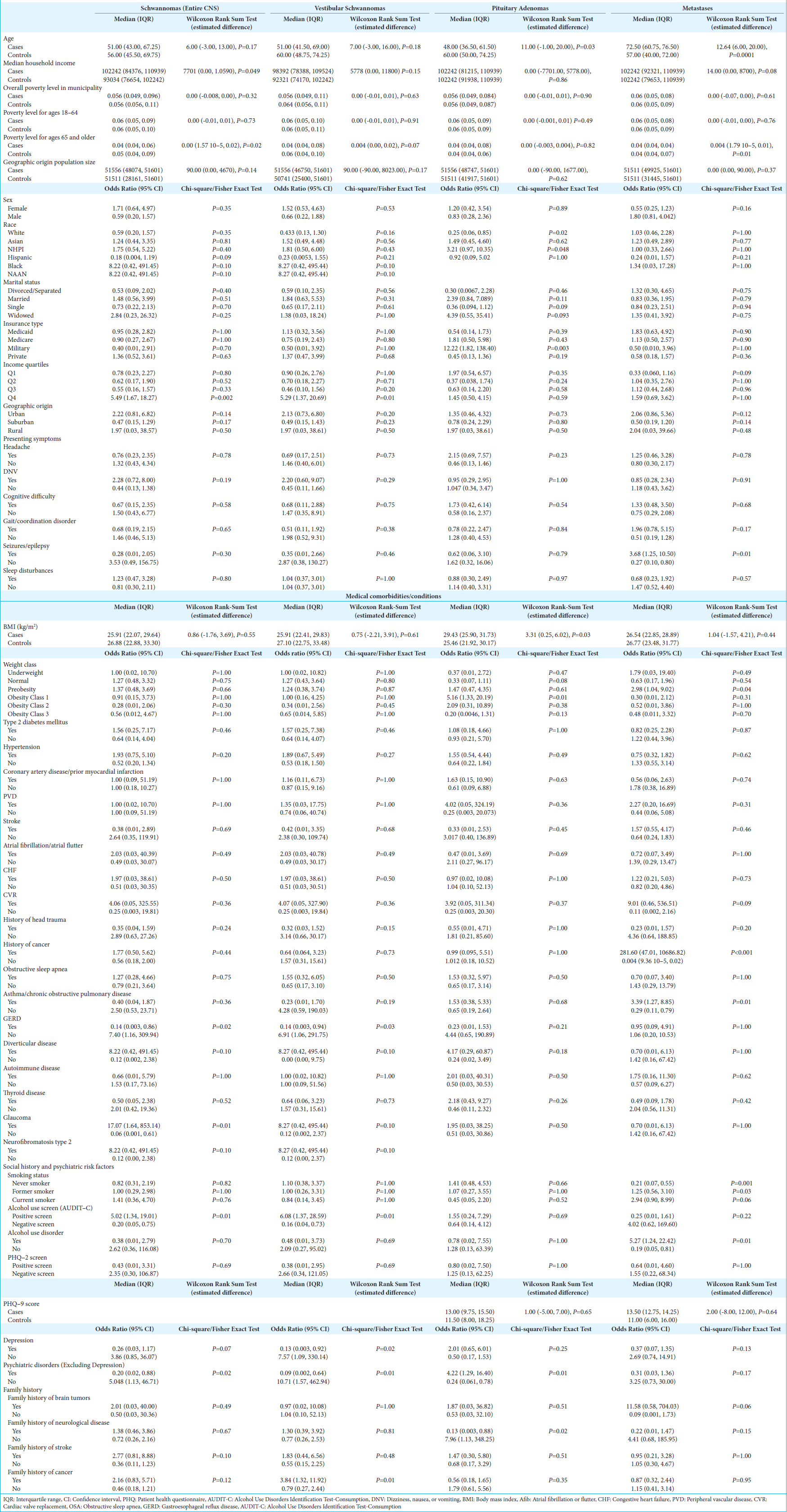
In the cohort, 8.0% of the cases (n = 26) cases were schwannomas, of which 84.6% were vestibular schwannomas (n = 22) [
Patients with schwannomas had significantly increased odds of being in the highest income quartile (5.49, 95% CI: 1.67, 18.27, P = 0.002) and from municipalities with a lower proportion of the populace below the poverty line (0.00, 95% CI: 1.57 × 10−5, 0.02; P = 0.02).
Patients with schwannomas had increased odds of glaucoma (17.07, 95% CI: 1.64, 853.14, P = 0.006), while vestibular schwannomas specifically had increased odds of having a positive alcohol screen (6.23, 95% CI: 1.69, 22.88, P = 0.006) and family history of cancer (3.89, 95% CI: 1.46, 10.36, P = 0.007). Patients with vestibular schwannomas also had decreased odds of depression/dysthymic disorder (0.13, 95% CI: 0.02, 1.03, P = 0.05) and other psychiatric disorders (0.09, 95% CI: 0.01, 0.72, P = 0.023). Meanwhile, vestibular schwannoma patients exhibited decreased odds of gastroesophageal reflux disease (GERD) (0.14, 95% CI: 0.0034, 0.94, P = 0.03).
On conducting multivariable analysis, the following variables were identified as the best predictors of vestibular schwannoma diagnosis, including being from the highest income quartile (24.88, 95% CI: 2.14, 289.14; P = 0.01), presenting with DNV (5.75, 95% CI: 1.13, 29.23; P = 0.04), and having a family history of cancer (14.66, 95% CI: 2.25, 95.30; P = 0.005).
Tumor size
NHPI patients exhibited significantly larger tumor size at the time of diagnosis (14.00, 95% CI: 1.00, 26.00, P = 0.048) compared to Asians.
Pituitary adenomas
About 6.8% of the cohort (n = 22) had pituitary adenomas, with a median age at diagnosis at 48 years (IQR: 36.50, 61.50), 11.0 years younger than controls (95% CI: 1.00, 20.00; P = 0.03) and no sex predisposition [
Pituitary adenoma patients at diagnosis had a significantly higher median BMI, by 3.31 kg/m2 (95% CI: 0.25, 6.02; P = 0.03), with obesity class I resulting in a 5.16 fold (95% CI: 1.33, 20.19; P = 0.01) greater odds of diagnosis. Meanwhile, having a history of psychiatric disorder (excluding depression) increased the odds of a pituitary adenoma diagnosis by 4.22 fold (95% CI: 1.29, 16.40; P = 0.01).
Intracranial metastases
In the tumor cohort, there were 36 patients (11.1%) with intracranial metastases [
Tumor size
Although not statistically significant, Asian patients had significantly larger tumor sizes compared to NHPI (P = 0.05) [
DISCUSSION
Age and sex
Of the 323 tumors in the cohort, 49.2% were meningiomas (39.9% benign and 9.3% malignant), 12.1% gliomas (6.2% Grades I–III and 5.9% Grade IV), 11.1% intracranial metastases, 8.0% schwannomas (6.8% vestibular schwannomas), and 6.8% pituitary adenomas. These trends overall parallel those from the Central Brain Tumor Registry of the United States (CBTRUS), where meningiomas and gliomas were the most common primary intracranial lesions.[
The youngest age of diagnosis was among Grades I–III gliomas at 47.5 years (IQR: 31.5, 62.0), followed by pituitary adenomas at 48.0 (36.5, 61.5), vestibular schwannomas at 51.0 (41.5, 69.0), Grade IV gliomas at 59.0 (50.0, 70.5), benign meningiomas at 61.0 (50.0, 71.0), malignant meningiomas at 66.5 (49.0, 76.0), and metastases at 72.5 (60.8, 76.5). Although gliomas, schwannomas, meningiomas, and metastases had similar values to that of national datasets, the age of diagnosis for pituitary adenomas was younger than expected.[
Similar to the CBTRUS results, which found a 1.25 fold higher incidence rate of primary central nervous system tumors in females (26.31/100,000; males: 21.09/100,000), females in our Hawaiian cohort had a 1.44-fold higher odds of tumor diagnosis.[
Race/ethnicity
Collectively, Hispanic/Latino patients in Hawaii exhibited a significantly lower likelihood of primary intracranial tumor diagnosis than non-Hispanic patients, similar to observations in CBTRUS.[
In contrast to national datasets where NAAN had the lowest incidence rates of meningioma, NAAN in Hawaii had a 12.26 fold greater odds of benign meningioma diagnosis.[
For pituitary adenomas, NHPI had the highest odds of diagnosis at 3.21 (1.03, 10.35), followed by Asians at 1.49 (0.45, 4.60), Hispanics at 0.92 (0.09, 5.02) and Whites at 0.25 (0.06, 0.85); these trends parallel a previous nationwide study that found the highest incidence among Asians and Pacific Islanders, and the lowest among Whites.[
Finally, the observation of higher rates of glioma diagnosis among Whites persisted in Hawaii, with the additional finding that Asians had significantly reduced odds of glioma diagnosis.[
Socioeconomic variables
Of the tumors investigated, malignant meningiomas, schwannomas, pituitary adenomas, and intracranial metastases exhibited unique socioeconomic associations. Malignant meningiomas, schwannomas, and metastases were all more likely to be diagnosed in patients from households with greater median income, higher income quartiles, or municipalities with less poverty. For meningiomas, prior literature is inconclusive regarding the role of socioeconomic status: one investigation from Sweden found an increased incidence of meningiomas in women with higher socioeconomic status, while a second Swedish investigation found no association.[
The increased odds of brain tumors overall among Medicare patients may be due to an older age of diagnosis for brain tumors.[
Furthermore, among gliomas, the odds of diagnosis were significantly higher among married patients, likely yielding from spouses more likely to appreciate cognitive changes in a patient.[
Presenting symptoms
In the overall brain tumor cohort, seizures were the most common presenting symptom, a finding consistent with prior studies.[
Medical comorbidities
In the entire cohort, brain tumors overall were associated with decreased odds of GERD and increased odds of hypertension, traumatic brain injury, obesity, diverticular disease, and a prior history of cancer. However, after multivariable analysis, only the association with obesity and prior history of cancer remained significant among the general cohort.
On stratification, benign meningiomas exhibited a positive association with hypertension and obesity, findings consistent with another study in a multiethnic cohort, potentially linking metabolic syndrome with an increased risk of meningioma.[
Asthma was associated with reduced odds of glioblastoma diagnosis, a trend paralleling that of pediatric brain tumors, where T-cell mediated disorders result in reduced tumor frequency.[
The increased odds of glaucoma among schwannomas overall, as well as reduced odds of GERD among vestibular schwannomas and pituitary adenomas, have not been described in the literature. Of note, sensorineural hearing loss has been linked to an increased incidence of glaucoma; however, the etiology of the association remains unelucidated, and a link with schwannomas is unclear.
Psychiatric risk factors and social history
Our cohort did exhibit a correlation between a positive AUDIT-C screen with intracranial tumor diagnosis, contrary to prior studies that found no association between alcohol consumption and brain tumor risk.[
Smoking history was found only to be associated with intracranial metastases, most likely accounted for by the strong association between smoking and non-intracranial cancers.[
Pituitary adenomas were the only tumor type to exhibit a positive correlation with psychiatric history.[
Family history
Family history of cancer is an established risk factor for brain tumors, consistent with our overall cohort, as well as when stratified among gliomas and vestibular schwannomas.[
Tumor size at diagnosis associated with race
Several racial disparities were highlighted when examining tumor size at the time of diagnosis [
Limitations
Although this exploratory study identified several novel correlations, the findings should be considered in the context of several limitations. First, the study is retrospective and relies on accurate documentation by healthcare workers. The reliance on ICD codes for case ascertainment leads to susceptibility to administrative errors in data entry, meaning that cases could be inadvertently undetected. Furthermore, certain social history and psychiatric risk factors may be susceptible to recall bias or patient’s reluctance to disclose due to stigmatization of mental health. Finally, a limited sample size may have decreased statistical power.
CONCLUSION
This study identified several sociodemographic differences in intracranial tumors, which, in turn, may have implications for diagnosis, treatment, and healthcare policy. For benign meningiomas, gliomas, and vestibular schwannomas, NHPI presented with significantly larger tumor volumes at diagnosis than Whites and/or Asians. There were greater odds of diagnosis of benign meningiomas among NAAN, increased odds of diagnosis of gliomas among Whites (reduced among Asians), and increased odds of pituitary adenomas among NHPI (reduced among Whites). Affluence was associated with a diagnosis of malignant meningioma, vestibular schwannoma, and intracranial metastases. Hence, among brain tumors, there are key healthcare disparities that may implicate survival outcomes being linked to a patient’s sociodemographic background.
Authors’ contributions
All authors contributed equally to the development of this project and manuscript.
Availability of data and material
Data supporting this study can be made available upon reasonable request. Further data can be found in
Code availability
Code supporting this study can be made available on reasonable request.
Ethics approval
The Institutional Review Board approval was obtained before the study from the University of Hawaii, Office of Research Compliance (protocol number: 2020-01010).
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary material:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ahn S, Han K, Lee JE, Jeun SS, Park YM, Yang SH. Associations of general and abdominal obesity with the risk of glioma development. Cancers (Basel). 2021. 13: 2859
2. Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019. 16: 509-20
3. Alentorn A, Hoang-Xuan K, Mikkelsen T. Presenting signs and symptoms in brain tumors. Handb Clin Neurol. 2016. 134: 19-26
4. Almeida J, Costa J, Coelho P, Cea V, Galesio M, Noronha JP. Adipocyte proteome and secretome influence inflammatory and hormone pathways in glioma. Metab Brain Dis. 2019. 34: 141-52
5. Alther B, Mylius V, Weller M, Gantenbein AR. From first symptoms to diagnosis: Initial clinical presentation of primary brain tumors. Clin Transl Neurosci. 2020. 4: 17
6. Altuntaş SÇ, Evran M, Sert M, Tetiker T. Markers of metabolic syndrome in patients with pituitary adenoma: A case series of 303 patients. Horm Metab Res. 2019. 51: 709-13
7. Aluli NE. Prevalence of obesity in a Native Hawaiian population. Am J Clin Nutr. 1991. 53: 1556S-60
8. Anzalone CL, Glasgow AE, Van Gompel JJ, Carlson ML. Racial differences in disease presentation and management of intracranial meningioma. J Neurol Surg B Skull Base. 2019. 80: 555-61
9. Assi HI, Hilal L, Abu-Gheida I, Berro J, Sukhon F, Skaf G. Demographics and outcomes of meningioma patients treated at a tertiary care center in the Middle East. Clin Neurol Neurosurg. 2020. 195: 105846
10. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG, editors. World Health Organization. AUDIT: The alcohol use disorders identification test: Guidelines for use in primary health care. Geneva: World Health Organization; 2001. p.
11. Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br J Cancer. 2015. 112: 580-93
12. Barnholtz-Sloan JS, Maldonado JL, Williams VL, Curry WT, Rodkey EA, Barker FG. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol. 2007. 85: 171-80
13. Baumgartner ET, Grossmann B, Fuddy L. Hawaii’s near-universal health insurance--lessons learned. J Health Care Poor Underserved. 1993. 4: 194-202
14. Benson VS, Pirie K, Green J, Casabonne D, Beral V. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008. 99: 185-90
15. Blumenthal DT, Cannon-Albright LA. Familiality in brain tumors. Neurology. 2008. 71: 1015-20
16. Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D. Brain tumor epidemiology: Consensus from the brain tumor epidemiology consortium. Cancer. 2008. 113: 1953-68
17. Brokinkel B, Hess K, Mawrin C. Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: A systematic review. Neuro Oncol. 2017. 19: 1298-307
18. Buckingham RS, Cornforth LL, Whitwell KJ, Lee RB. Visual acuity, optical, and eye health readiness in the military. Mil Med. 2003. 168: 194-8
19. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med. 1998. 158: 1789-95
20. Chaichana KL, Chaichana KK, Olivi A, Weingart JD, Bennett R, Brem H. Surgical outcomes for older patients with glioblastoma multiforme: Preoperative factors associated with decreased survival. Clinical article. J Neurosurg. 2011. 114: 587-94
21. Chatterjee J, Sanapala S, Cobb O, Bewley A, Goldstein AK, Cordell E. Asthma reduces glioma formation by T cell decorin-mediated inhibition of microglia. Nat Commun. 2021. 12: 7122
22. Chen P, Aldape K, Wiencke JK, Kelsey KT, Miike R, Davis RL. Ethnicity delineates different genetic pathways in malignant glioma. Cancer Res. 2001. 61: 3949-54
23. Claus EB, Calvocoressi L, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M. Family and personal medical history and risk of meningioma. J Neurosurg. 2011. 115: 1072-7
24. Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: A test of social learning formulations. J Abnorm Psychol. 1988. 97: 218-30
25. Corona AP, Oliveira JC, Souza FP, Santana LV, Rêgo MA. Risk factors associated with vestibulocochlear nerve schwannoma: Systematic review. Braz J Otorhinolaryngol. 2009. 75: 593-615
26. Curry WT, Barker FG 2nd. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009. 93: 25-39
27. Dachs GU, Currie MJ, McKenzie F, Jeffreys M, Cox B, Foliaki S. Cancer disparities in indigenous Polynesian populations: Māori, Native Hawaiians, and Pacific people. Lancet Oncol. 2008. 9: 473-84
28. Das A, Tan WL, Teo J, Smith DR. Glioblastoma multiforme in an Asian population: Evidence for a distinct genetic pathway. J Neurooncol. 2002. 60: 117-25
29. Dubrow R, Darefsky AS. Demographic variation in incidence of adult glioma by subtype, United States, 1992-2007. BMC Cancer. 2011. 11: 325
30. Escarce JJ, Kapur K, editors. Access to and quality of health care. Washington, DC: National Academies Press; 2006. p.
31. Ferrer VP, Moura Neto V, Mentlein R. Glioma infiltration and extracellular matrix: Key players and modulators. Glia. 2018. 66: 1542-65
32. Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007. 25: 867-90 vii
33. Foley RW, Shirazi S, Maweni RM, Walsh K, McConn Walsh R, Javadpour M. Signs and symptoms of acoustic neuroma at initial presentation: An exploratory analysis. Cureus. 2017. 9: e1846
34. Galeone C, Malerba S, Rota M, Bagnardi V, Negri E, Scotti L. A meta-analysis of alcohol consumption and the risk of brain tumours. Ann Oncol. 2013. 24: 514-23
35. Ghaffari-Rafi A, Mehdizadeh R, Ghaffari-Rafi S, Castillo JA, Rodriguez-Beato FY, Leon-Rojas J. Demographic and socioeconomic disparities of pituitary adenomas and carcinomas in the United States. J Clin Neurosci. 2022. 98: 96-103
36. Ghaffari-Rafi A, Mehdizadeh R, Ghaffari-Rafi S, Leon-Rojas J. Demographic and socioeconomic disparities of benign and malignant spinal meningiomas in the United States. Neurochirurgie. 2021. 67: 112-8
37. Ghaffari-Rafi A, Mehdizadeh R, Ko AW, Ghaffari-Rafi S, Leon-Rojas J. Demographic and socioeconomic disparities of benign cerebral meningiomas in the United States. J Clin Neurosci. 2021. 86: 122-8
38. Ghaffari-Rafi A, Samandouras G. Effect of treatment modalities on progression-free survival and overall survival in molecularly subtyped World Health Organization grade II diffuse gliomas: A systematic review. World Neurosurg. 2020. 133: 366-80.e2
39. Grimes DA, Schulz KF. Compared to what?. Finding controls for case-control studies. Lancet. 2005. 365: 1429-33
40. Hale AT, Wang L, Strother MK, Chambless LB. Differentiating meningioma grade by imaging features on magnetic resonance imaging. J Clin Neurosci. 2018. 48: 71-5
41. Harding NJ, Birch JM, Hepworth SJ, McKinney PA. Atopic dysfunction and risk of central nervous system tumours in children. Eur J Cancer. 2008. 44: 92-9
42. Hess K, Spille DC, Adeli A, Sporns PB, Brokinkel C, Grauer O. Brain invasion and the risk of seizures in patients with meningioma. J Neurosurg. 2018. 130: 789-96
43. Hodges TR, Labak CM, Mahajan UV, Wright CH, Wright J, Cioffi G. Impact of race on care, readmissions, and survival for patients with glioblastoma: An analysis of the National Cancer Database. Neurooncol Adv. 2021. 3: vdab040
44. Infante-Rivard C, Roncarolo F, Doucette K. Reliability of cancer family history reported by parents in a case-control study of childhood leukemia. Cancer Causes Control. 2012. 23: 1665-72
45. Inskip PD, Tarone RE, Hatch EE, Wilcosky TC, Fine HA, Black PM. Sociodemographic indicators and risk of brain tumours. Int J Epidemiol. 2003. 32: 225-33
46. Johnson DR, Olson JE, Vierkant RA, Hammack JE, Wang AH, Folsom AR. Risk factors for meningioma in postmenopausal women: Results from the Iowa Women’s Health Study. Neuro Oncol. 2011. 13: 1011-9
47. Kendel F, Wirtz M, Dunkel A, Lehmkuhl E, Hetzer R, RegitzZagrosek V. Screening for depression: Rasch analysis of the dimensional structure of the PHQ-9 and the HADS-D. J Affect Disord. 2010. 122: 241-6
48. Kotagal V, Langa KM, Plassman BL, Fisher GG, Giordani BJ, Wallace RB. Factors associated with cognitive evaluations in the United States. Neurology. 2015. 84: 64-71
49. Kriston L, Hölzel L, Weiser AK, Berner MM, Härter M. Meta-analysis: Are 3 questions enough to detect unhealthy alcohol use?. Ann Intern Med. 2008. 149: 879-88
50. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001. 16: 606-13
51. Kroenke K, Spitzer RL, Williams JB, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: A systematic review. Gen Hosp Psychiatry. 2010. 32: 345-59
52. Lazar M, Davenport L. Barriers to health care access for low income families: A review of literature. J Community Health Nurs. 2018. 35: 28-37
53. Malmer B, Henriksson R, Grönberg H. Familial brain tumoursgenetics or environment? A nationwide cohort study of cancer risk in spouses and first-degree relatives of brain tumour patients. Int J Cancer. 2003. 106: 260-3
54. Mathew GD, Facer GW, Suh KW, Houser OW, O’Brien PC. Symptoms, findings, and methods of diagnosis in patients with acoustic neuroma. Laryngoscope. 1978. 88: 1893-903 1921
55. McDowell BD, Wallace RB, Carnahan RM, Chrischilles EA, Lynch CF, Schlechte JA. Demographic differences in incidence for pituitary adenoma. Pituitary. 2011. 14: 23-30
56. McHugh ML. The chi-square test of independence. Biochem Med (Zagreb). 2013. 23: 143-9
57. Melkonian SC, Weir HK, Jim MA, Preikschat B, Haverkamp D, White MC. Incidence of and trends in the leading cancers with elevated incidence among American Indian and Alaska Native Populations, 2012-2016. Am J Epidemiol. 2021. 190: 528-38
58. Mochizuki S, Iwadate Y, Namba H, Yoshida Y, Yamaura A, Sakiyama S. Homozygous deletion of the p16/MTS-1/CDKN2 gene in malignant gliomas is infrequent among Japanese patients. Int J Oncol. 1999. 15: 983-9
59. Muskens IS, Wu AH, Porcel J, Cheng I, Le Marchand L, Wiemels JL. Body mass index, comorbidities, and hormonal factors in relation to meningioma in an ethnically diverse population: The Multiethnic Cohort. Neuro Oncol. 2019. 21: 498-507
60. Navas-Acién A, Pollán M, Gustavsson P, Plato N. Occupation, exposure to chemicals and risk of gliomas and meningiomas in Sweden. Am J Ind Med. 2002. 42: 214-27
61. Nayak L, Iwamoto FM. Primary brain tumors in the elderly. Curr Neurol Neurosci Rep. 2010. 10: 252-8
62. Osawa T, Tosaka M, Nagaishi M, Yoshimoto Y. Factors affecting peritumoral brain edema in meningioma: Special histological subtypes with prominently extensive edema. J Neurooncol. 2013. 111: 49-57
63. Ostrom QT, Adel Fahmideh M, Cote DJ, Muskens IS, Schraw JM, Scheurer ME. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019. 21: 1357-75
64. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019. 21: v1-100
65. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021. 23: iii1-105
66. Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018. 4: 1254-62
67. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020. 22: iv1-96
68. Pan IW, Ferguson SD, Lam S. Patient and treatment factors associated with survival among adult glioblastoma patients: A USA population-based study from 2000-2010. J Clin Neurosci. 2015. 22: 1575-81
69. Park JS, Sade B, Oya S, Kim CG, Lee JH. The influence of age on the histological grading of meningiomas. Neurosurg Rev. 2014. 37: 425-9 discussion 429
70. Pengili T, Hashorva A, Prifti I, Lici M, Balani E. Cases where pituitary tumor is presented first with psychiatric signs are very rare. Eur Psychiatry. 2017. 41: S483
71. Radley D, Baumgartner J, Collins S, Zephyrin L, Schneider E, editors. Achieving racial and ethnic equity in US health care: A scorecard of state performance. New York: The Commonwealth Fund; 2021. p.
72. Reinert DF, Allen JP. The alcohol use disorders identification test: An update of research findings. Alcohol Clin Exp Res. 2007. 31: 185-99
73. Reznitsky M, Petersen MM, West N, Stangerup SE, CayéThomasen P. Epidemiology of vestibular schwannomas-prospective 40-year data from an unselected national cohort. Clin Epidemiol. 2019. 11: 981-6
74. Robertson JT, Gunter BC, Somes GW. Racial differences in the incidence of gliomas: A retrospective study from Memphis, Tennessee. Br J Neurosurg. 2002. 16: 562-6
75. Schmid C, Goede DL, Hauser RS, Brändle M. Increased prevalence of high Body Mass Index in patients presenting with pituitary tumours: Severe obesity in patients with macroprolactinoma. Swiss Med Wkly. 2006. 136: 254-8
76. Schoemaker MJ, Swerdlow AJ, Auvinen A, Christensen HC, Feychting M, Johansen C. Medical history, cigarette smoking and risk of acoustic neuroma: An international case-control study. Int J Cancer. 2007. 120: 103-10
77. Schüz J, Steding-Jessen M, Hansen S, Stangerup SE, CayéThomasen P, Johansen C. Sociodemographic factors and vestibular schwannoma: A Danish nationwide cohort study. Neuro Oncol. 2010. 12: 1291-9
78. Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M, Malmer B. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009. 41: 899-904
79. Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015. 72: 3323-42
80. Tamiya T, Ono Y, Matsumoto K, Ohmoto T. Peritumoral brain edema in intracranial meningiomas: Effects of radiological and histological factors. Neurosurgery. 2001. 49: 1046-51
81. R Core Team. R: A language and environment for statistical computing. Available From: https://www.R-project-org [Last accessed on 2024 Mar 08].
82. Tindle HA, Stevenson Duncan M, Greevy RA, Vasan RS, Kundu S, Massion PP. Lifetime smoking history and risk of lung cancer: Results from the Framingham heart study. J Natl Cancer Inst. 2018. 110: 1201-7
83. Turner MC, Krewski D, Armstrong BK, Chetrit A, Giles GG, Hours M. Allergy and brain tumors in the INTERPHONE study: Pooled results from Australia, Canada, France, Israel, and New Zealand. Cancer Causes Control. 2013. 24: 949-60
84. Weaver JL, McAlister WH. Vision readiness of the reserve forces of the U.S. Army. Mil Med. 2001. 166: 64-6
85. Weitzner MA, Kanfer S, Booth-Jones M. Apathy and pituitary disease: It has nothing to do with depression. J Neuropsychiatry Clin Neurosci. 2005. 17: 159-66
86. White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health. 2014. 104: S377-87
87. Whittle IR. The dilemma of low grade glioma. J Neurol Neurosurg Psychiatry. 2004. 75: ii31-6
88. Wiedmann MK, Brunborg C, Di Ieva A, Lindemann K, Johannesen TB, Vatten L. Overweight, obesity and height as risk factors for meningioma, glioma, pituitary adenoma and nerve sheath tumor: A large population-based prospective cohort study. Acta Oncol. 2017. 56: 1302-9
89. Wiegand DA, Fickel V. Acoustic neuroma--the patient’s perspective: Subjective assessment of symptoms, diagnosis, therapy, and outcome in 541 patients. Laryngoscope. 1989. 99: 179-87
90. Wigertz A, Lönn S, Hall P, Feychting M. Non-participant characteristics and the association between socioeconomic factors and brain tumour risk. J Epidemiol Community Health. 2010. 64: 736-43
91. Wigertz A, Lönn S, Schwartzbaum J, Hall P, Auvinen A, Christensen HC. Allergic conditions and brain tumor risk. Am J Epidemiol. 2007. 166: 941-50
92. Wilcox JA, Naranjo J. Psychiatric manifestations of pituitary tumors. Psychosomatics. 1997. 38: 396-7
93. Wilcoxon F. Individual comparisons by ranking methods. Biom Bull. 1945. 1: 80-3
94. Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y, Decker PA. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009. 41: 905-8
95. Wrensch M, Lee M, Miike R, Newman B, Barger G, Davis R. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. 1997. 145: 581-93