- Department of Neurosurgery, Hospital Prof Juan P. Garrahan, Capital, Argentina
Correspondence Address:
Yamila Basilotta Marquez, Department of Neurosurgery, Hospital Prof Juan P. Garrahan, Capital, Argentina.
DOI:10.25259/SNI_238_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yamila Basilotta Marquez, Juan Medina, Diego Fernando Velasco Sanchez, Romina Argañaraz. Diffuse midline H3K27-altered glioma in an atypical location mimicking a medulloblastoma. 30-May-2025;16:215
How to cite this URL: Yamila Basilotta Marquez, Juan Medina, Diego Fernando Velasco Sanchez, Romina Argañaraz. Diffuse midline H3K27-altered glioma in an atypical location mimicking a medulloblastoma. 30-May-2025;16:215. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13589
Abstract
Background: Diffuse midline glioma (DMG) with H3 histones in lysine 27 (H3K27) mutation is an aggressive central nervous system tumor that primarily affects children. It often presents with nonspecific neurological symptoms and can mimic other posterior fossa tumors, such as medulloblastoma, on imaging. Due to its poor prognosis and rapid progression, early recognition and accurate diagnosis are crucial for patient management.
Case Description: We present the case of a 10-year-old girl who developed progressively worsening neurological symptoms, raising suspicion of a posterior fossa tumor. Initial magnetic resonance imaging findings suggested a diagnosis of medulloblastoma. However, after surgical resection, pathological analysis confirmed the presence of a DMG with an H3K27 mutation. Despite the successful resection of a substantial portion of the tumor, the disease progressed rapidly, with tumor dissemination occurring within six months of diagnosis.
Conclusion: This case highlights the importance of considering DMG, particularly with H3K27 alterations, as a differential diagnosis in posterior fossa tumors. The presence of these genetic mutations significantly impacts both treatment decisions and prognosis. The variability in clinical presentation and tumor morphology associated with DMG underscores the need for thorough evaluation to optimize treatment strategies and further our understanding of this complex entity.
Keywords: Diffuse midline glioma, H3 histones in lysine 27-altered, Medulloblastoma, Posterior fossa tumor
INTRODUCTION
Diffuse midline H3 histones in lysine 27 (H3k27) gliomas belong to high-grade diffuse tumors according to the new classifications of the World Health Organization (WHO).[
These tumors present a different phenotype and can imitate other kinds of neoplasms such as medulloblastomas, gangliogliomas, and low-grade gliomas, among others.[
CASE DESCRIPTION
A 10-year-old patient, female, with no previous history, presents with progressive neurological symptoms for 15 days. She presented with headache, broad-based ataxic gait, and poor coordination mainly on the left side. The vital signs were in the normal range, and the neurologic exam revealed equal pupils with light reactivity, no cranial nerve deficit, and good motor strength throughout all extremities. The initial computed tomography (CT) scan revealed a posterior fossa tumor in the left cerebellar hemisphere with ventricular dilation [
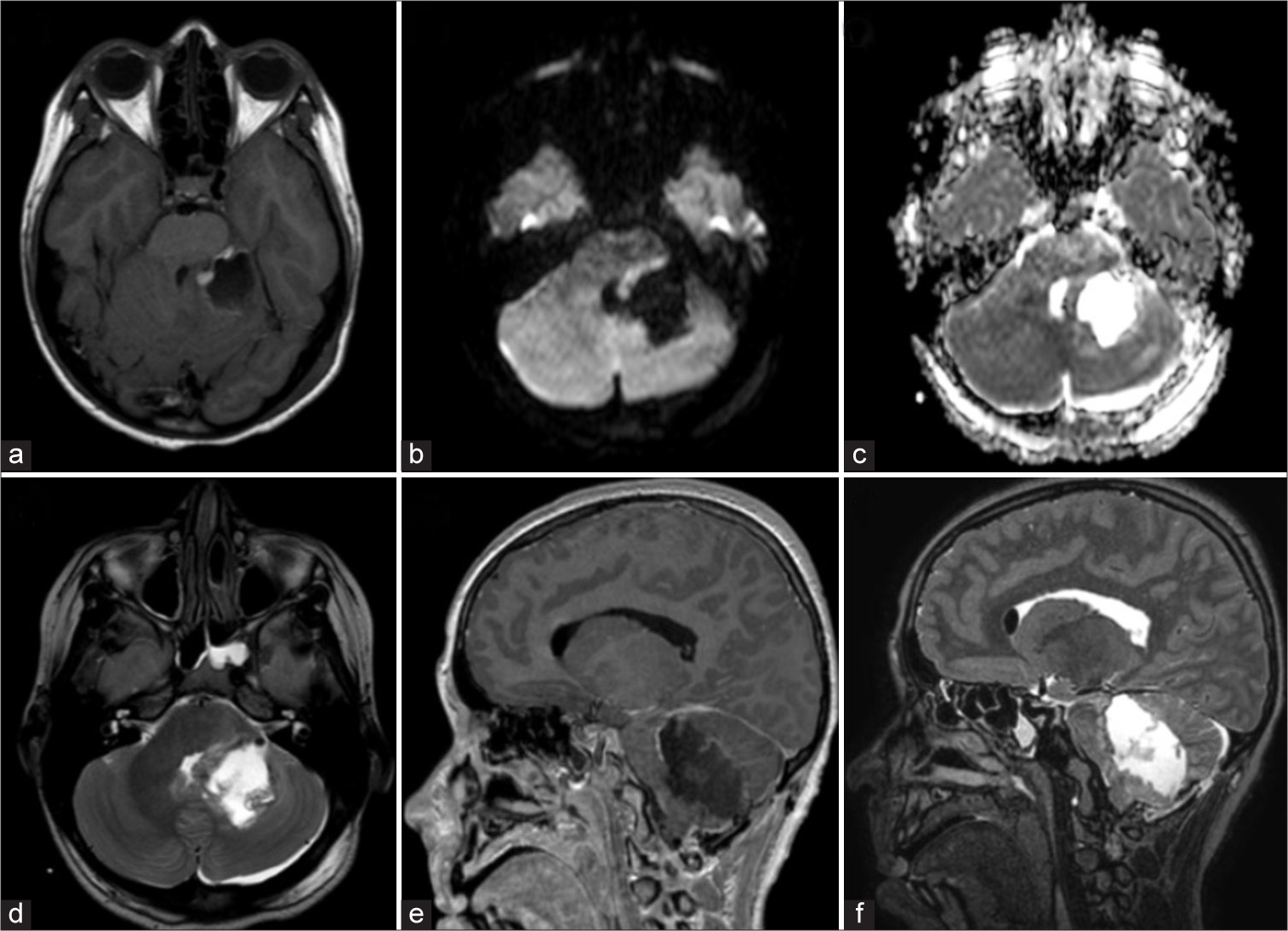
Figure 1:
(a) Axial computed tomography scan showing a solid mass in the left cerebellar hemisphere and vermis, isodense and mostly homogeneous, with a peripheral hypodense halo. (b and c) Axial magnetic resonance imaging (MRI) brain scans using diffusion-weighted imaging and ADC map, respectively, demonstrating a high signal in the diffusion-weighted sequence and a corresponding low signal in the apparent diffusion coefficient. (d) Axial MRI in T2 sequence revealing a hyperintense signal, indicating extension to the ipsilateral cerebellopontine angle. (e) Sagittal MRI after contrast administration, showing heterogeneous enhancement and caudal displacement of the cerebellar tonsils through the foramen magnum (pseudo Chiari), ventral to the brainstem. (f) Sagittal T2 MRI displaying adjacent cerebellar, pontine, and bulbar parenchyma with diffuse borders and hyperintense signal, consistent with vasogenic edema.
An external ventricular drainage was placed with clinical improvement. Afterward, she underwent tumor resection. The intraoperative pathology informed a high-grade glial tumor. In the immediate postoperative period, she was extubated but then required assisted ventilation due to her being unable to maintain the airway. On the same day, an magnetic resonance imaging (MRI) showed a large surgical resection [
Figure 2:
(a) Axial magnetic resonance imaging (MRI) with contrast revealing the surgical cavity with residual tumor in close proximity to the brainstem. (b and c) Diffusion-weighted imaging and ADC map highlighting the tumor remnant at the superior medial margin of the surgical cavity, measuring approximately 8 × 7 mm, without clear diffusion restriction. (d) Axial T2 MRI showing free cisterns, cerebellar spaces, and vasogenic edema in the bulbomedullary region. (e and f) Sagittal MRI in T1 and T2 sequences, respectively, illustrating the postoperative cavity.
The definitive pathology exam informed the cerebellar laminae were infiltrated by an atypical proliferation, of glial aspect, highly cellular, formed by hyperchromatic nuclei, round and pleomorphic, with some polylobulated and multinucleate forms. These cells were disposed anarchically around necrotic foci. Numerous atypical mitoses and vascular hyperplasia were found. The immunohistochemistry showed a +glial fibrillary acidic protein, +Olig2, multifocal +Synaptophysin, positive neurofilament in isolated cells, loss of nuclear expression of HK27me3, positivity for H3K27M, loss of nuclear expression of A TRX and a cellular proliferation index (ki67) of 50-60%. In the molecular study, the H3F3A K27M mutation was identified. The diagnosis was a diffuse midline glioma (DMG) with an alteration of the H3K27, grade 4 according to the WHO.
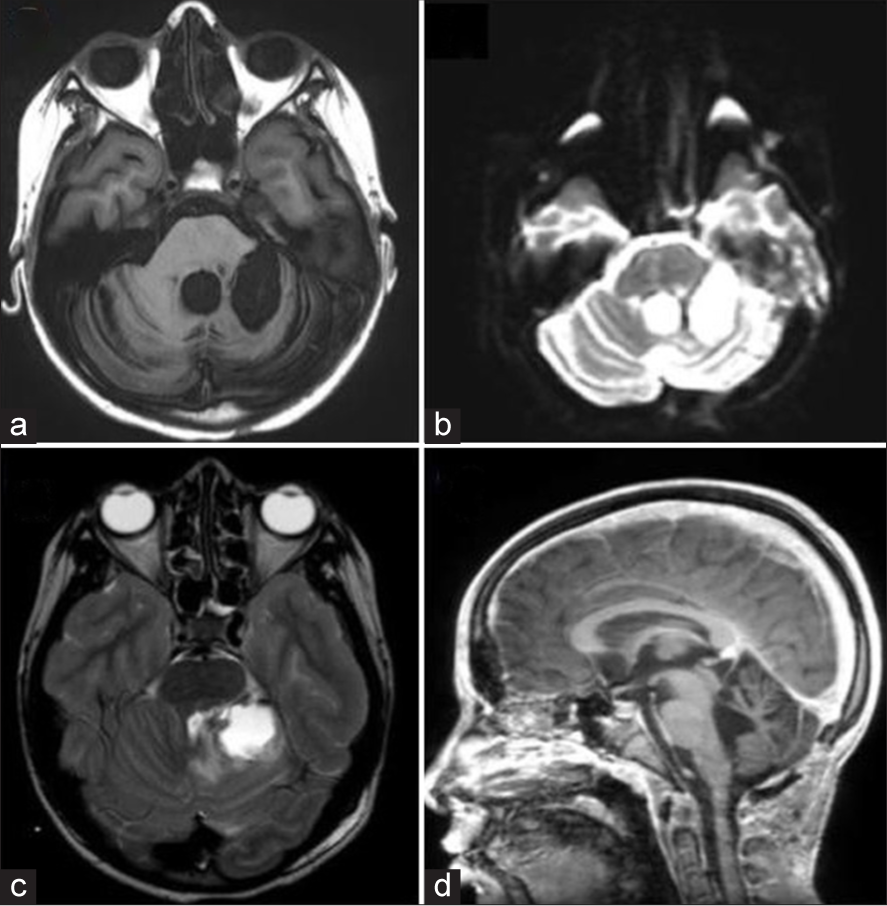
The patient received treatment with brain stem radiotherapy of 5500 cGy. She was discharged after 2 months [
Figure 3:
(a) Axial magnetic resonance imaging (MRI) post-radiation therapy, showing no evidence of tumor recurrence. (b) Diffusion MRI indicating no lesions with diffusion restriction. (c) T2-weighted MRI displaying postoperative changes distant from the surgical site. (d) Sagittal MRI from the distant postoperative period, revealing preserved brainstem spaces without tonsillar descent.
Figure 4:
(a) Axial brain CT scan showing a hyperdense intraventricular image, predominantly in the right ventricle. (b and c) Axial T1-weighted magnetic resonance imaging (MRI) with contrast, demonstrating an extensive heterogeneous tumor with intraventricular contrast enhancement. (d) Diffusion MRI revealing a lesion with diffusion restriction. (e) WTI sequence showing signs of hemorrhage in the right lateral ventricle. (f) Axial T1-weighted MRI with contrast revealing a nodular lesion adjacent to the midbrain. (g) Diffusion MRI indicating restricted diffusion in the aforementioned lesion. (h) Diffusion sequence image showing the surgical cavity.
DISCUSSION
Diffuse midline glioma de alto grado (DMHG) are aggressive tumors with an ominous prognosis. Their usual location is in the midline structures of the brain.[
Histones are proteins of the nucleosome and modifications of them can alter their interaction with DNA. The methylation of the H3K27 is found in the heterochromatin and its function is related to the silencing of genes.[
The bibliography suggests that independent of the location, these tumors have a poor prognosis due to their aggressiveness and the possibility of generating extracranial metastasis.[
This highlights the need to deepen the study of this entity to understand the importance of the presence of the H3K27 alteration in unusual cases. Even though in this case the patient passed away despite optimal therapy, many cases have demonstrated heterogeneity in this entity, both from the biological and the clinical standpoint. That is why we believe that the mere presence of the alteration is not enough to categorize this tumor as grade 4. This entity can have many locations and morphological phenotypes, or even different survival rates.[
There is a clear need for more investigation to comprehend the clinical implications and the possible pharmacological role of the H3K27 alteration, not only in midline tumors.
CONCLUSION
DMHG are aggressive tumors that can have multiple locations, morphological phenotypes, and different survival rates. We present an atypical case that shows we need more studies to comprehend the repercussions of the presence of the H3K27 alteration.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al Sharie S, Talafha M, Abu Laban D, Al Awabdeh T, Al-Mousa A, Al-Masri N. H3 K27M-mutant diffuse midline glioma with osseous metastases: A case report and a literature review. Clin Neuropathol. 2022. 41: 263-70
2. Fujioka Y, Hata N, Hatae R, Suzuki SO, Sangatsuda Y, Nakahara Y. A case of diffuse midline glioma, H3 K27M mutant mimicking a hemispheric malignant glioma in an elderly patient. Neuropathology. 2022. 40: 99-103
3. Gao Y, Feng YY, Yu JH, Li QC, Qiu XS, Wang EH. Diffuse midline gliomas with histone H3-K27M mutation: A rare case with PNET-like appearance and neuropil-like islands. Neuropathology. 2018. 38: 165-70
4. Gessi M, Capper D, Sahm F, Huang K, von Deimling A, Tippelt S. Evidence of H3 K27M mutations in posterior fossa ependymomas. Acta Neuropathol. 2016. 132: 635-7
5. Gianno F, Giovannoni I, Cafferata B, Diomedi-Camassei F, Minasi S, Barresi S. Paediatric-type diffuse high-grade gliomas in the 5th CNS WHO Classification. Pathologica. 2022. 114: 422-35
6. Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018. 20: 123-31
7. Kraus TF, Machegger L, Pöppe J, Zellinger B, Dovjak E, Schlicker HU. Diffuse midline glioma of the cervical spinal cord with H3 K27M genotype phenotypically mimicking anaplastic ganglioglioma: A case report and review of the literature. Brain Tumor Pathol. 2020. 37: 89-94
8. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, FigarellaBranger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
9. Morita S, Nitta M, Muragaki Y, Komori T, Masui K, Maruyama T. Brainstem pilocytic astrocytoma with H3 K27M mutation: Case report. J Neurosurg. 2018. 129: 593-7
10. Onishi S, Ohba S, Kuraoka K, Kurashige T, Sugiyama K, Yamasaki F. Molecular and clinical characterization of H3 K27M-mutant “non-midline” glioblastoma: A case report and literature review. Neurocirugía (Astur Engl Ed). 2022. 33: 356-60
11. Pan MR, Hsu MC, Chen LT, Hung WC. Orchestration of H3K27 methylation: Mechanisms and therapeutic implication. Cell Mol Life Sci. 2018. 75: 209-23
12. Sumi K, Shijo K, Igarashi T, Yamamuro S, Sasano M, Oshima H. Tectal low-grade glioma with H3 K27M mutation. World Neurosurg. 2020. 141: 91-100
13. Valério E, Alves de Castro JV, Carraro DM, Torrezan GT, Kulikowski LD, Costa FD. Beyond midline: Diffuse hemispheric glioma, H3 K27M-mutant with aggressive behavior. J Neuropathol Exp Neurol. 2022. 81: 381-3
14. Vallero SG, Bertero L, Morana G, Sciortino P, Bertin D, Mussano A. Pediatric diffuse midline glioma H3K27-altered: A complex clinical and biological landscape behind a neatly defined tumor type. Front Oncol. 2023. 12: 1082062
15. Wang L, Li Z, Zhang M, Piao Y, Chen L, Liang H. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol. 2018. 78: 89-96