- Department of Neurosurgery, Funabashi Municipal Medical Center, Funabashi City, Japan
Correspondence Address:
Kotaro Ueda, Department of Neurosurgery, Funabashi Municipal Medical Center, Funabashi City, Japan.
DOI:10.25259/SNI_950_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kotaro Ueda, Jun Niimi, Taiki Sako, Kosuke Ando, Kenta Tasaka, Fumio Nemoto, Kazumi Hatayama, Hiromichi Naito. Direct carotid-cavernous fistula completely treated with a small number of coils by combined transarterial and transvenous embolization: A case report. 17-Jan-2025;16:14
How to cite this URL: Kotaro Ueda, Jun Niimi, Taiki Sako, Kosuke Ando, Kenta Tasaka, Fumio Nemoto, Kazumi Hatayama, Hiromichi Naito. Direct carotid-cavernous fistula completely treated with a small number of coils by combined transarterial and transvenous embolization: A case report. 17-Jan-2025;16:14. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13343
Abstract
Background: Endovascular treatment options for direct carotid-cavernous fistula (CCF) include transarterial or transvenous embolization with detachable coils and balloons, parent artery occlusion, or the use of flow-diverting stents across the fistula. Although combined transarterial and transvenous embolization is uncommon, it can be advantageous. We present a case of direct CCF treated successfully with a combined approach using a minimal number of detachable coils.
Case Description: A 33-year-old female presented with tinnitus and headache following cesarean delivery and was transferred to our hospital. Cerebral angiography revealed a high-flow shunt from the superior lateral wall of the left cavernous internal carotid artery directly into the cavernous sinus, with a 3.5 × 2.8 mm shunted pouch. A diagnosis of direct CCF was confirmed. To maximize the packing density within the shunted pouch and to manage various situations during embolization, a combined transarterial and transvenous approach was utilized. Complete obliteration of the shunt was achieved without complications using only four detachable coils. The patient was discharged on postoperative day 3 with a modified Rankin Scale score of 0, and there has been no recurrence during the 6-month follow-up.
Conclusion: Direct CCF cases are relatively rare and complex to treat. This case illustrates practical strategies and considerations for achieving complete shunt obliteration with minimal intervention, highlighting the effectiveness of combined transarterial and transvenous embolization.
Keywords: Combined, Detachable coil, Direct carotid-cavernous fistula, Transarterial embolization, Transvenous embolization
INTRODUCTION
Direct carotid-cavernous fistula (CCF) is a relatively uncommon vascular condition characterized by a high-flow direct shunt between the internal carotid artery (ICA) and the cavernous sinus. Traumatic injury remains the primary cause, and other etiologies include the rupture of cavernous ICA aneurysms, connective tissue disorders such as Ehlers–Danlos syndrome, fibromuscular dysplasia, Marfan syndrome, as well as iatrogenic causes following endovascular intervention or transsphenoidal surgery.[
Endovascular approaches for treating direct CCF have been widely reported, including embolization with detachable coils or balloons (though the latter are not approved in Japan), parent artery occlusion, and deploying flow-diverting stents across the fistula site.[
CASE PRESENTATION
History and examination
A 33-year-old female with no prior medical history presented with tinnitus and headache following a cesarean delivery. She visited the neurosurgery department at a previous hospital 2 months after symptom onset. Magnetic resonance angiography (MRA) revealed abnormal blood flow signals in the cavernous sinus, and she was transferred to our hospital.
On initial examination, her blood pressure was 153/103 mmHg, pulse rate was 80 bpm, and she scored 15 on the Glasgow Coma Scale. Physical examination revealed left conjunctival hyperemia, and a vascular bruit was noted in the left postauricular lesion. However, there were no signs of pulsatile proptosis, diplopia, or visual disturbances.
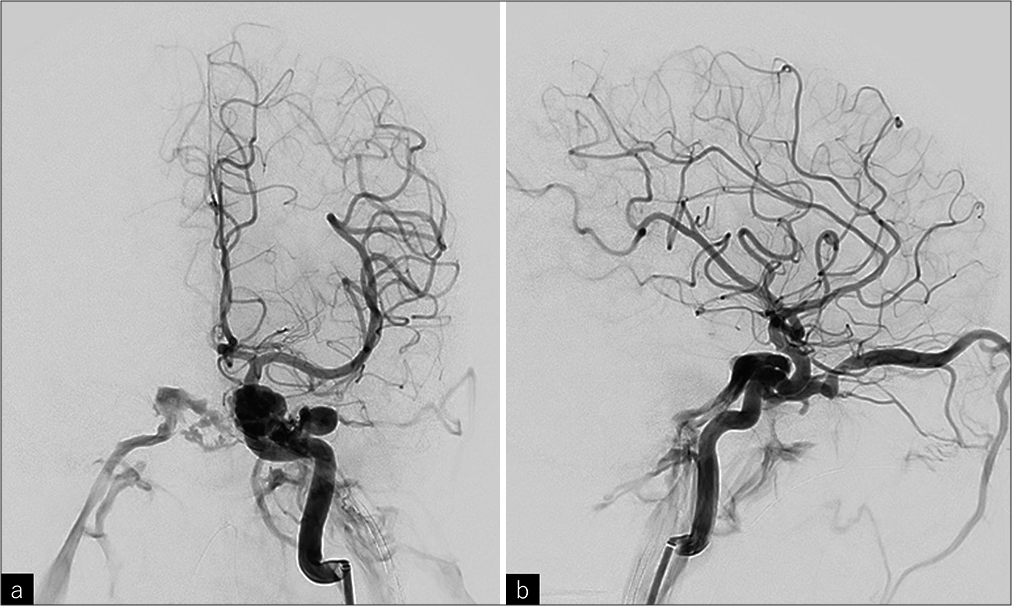
Time-of-flight MRA demonstrated hyperintensity in the cavernous sinus, bilateral superior ophthalmic veins, bilateral inferior petrosal sinuses, and the left superficial middle cerebral vein. Fluid-attenuated inversion recovery imaging showed no edematous changes in the cerebral parenchyma. Cerebral angiography confirmed a high-flow shunt from the superior lateral wall of the left cavernous ICA directly into the left cavernous sinus, with a shunted pouch measuring 3.5 × 2.8 mm [
Treatment
Our approach involved combining transarterial and transvenous embolization to maximize packing density within the shunted pouch, enabling it to handle the various situations during embolization.
Figure 1:
The left internal carotid artery (ICA) angiography revealed a high-flow shunt from the superior lateral wall of the left cavernous ICA directly into the left cavernous sinus. The shunted flow drained from the cavernous sinus into the bilateral superior ophthalmic veins and bilateral inferior petrosal sinuses, with reflux into the left superficial middle cerebral vein. (a) Posteroanterior view, (b) lateral view.
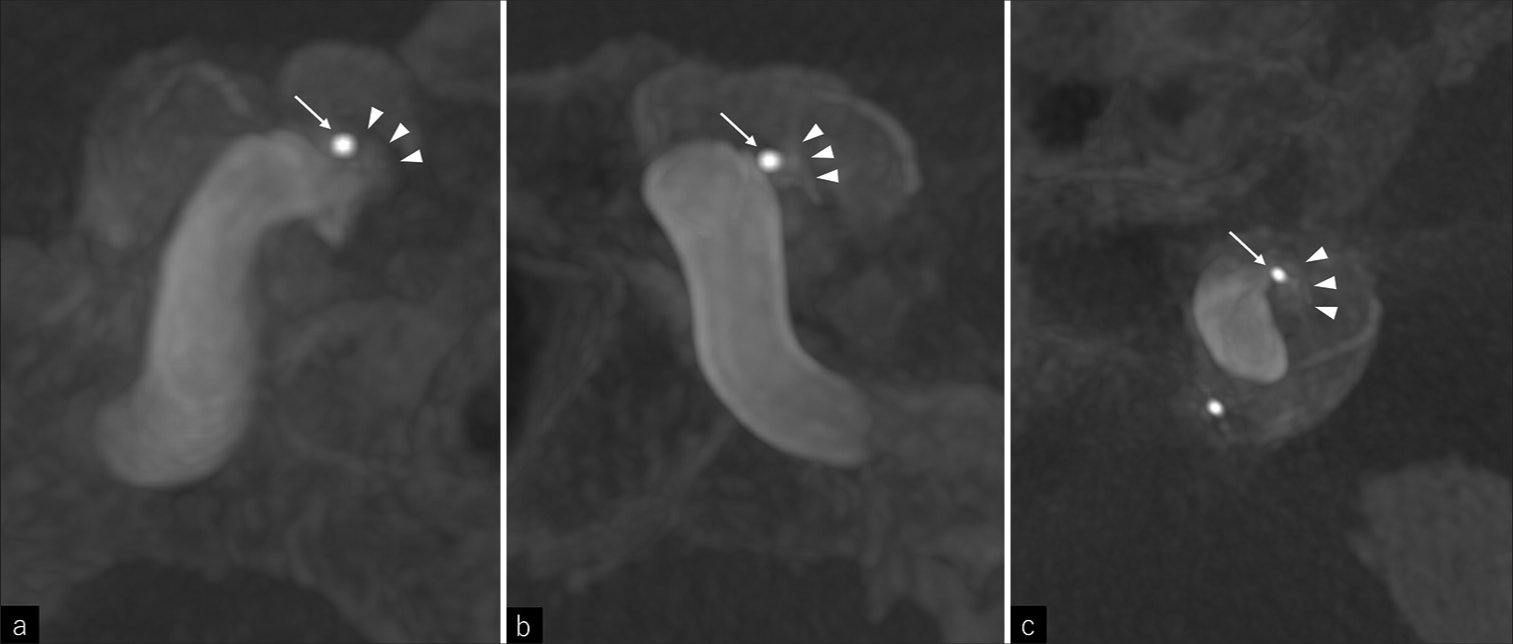
Under general anesthesia, an 8Fr long sheath, a 6Fr long sheath, and a 4Fr short sheath were inserted into the right femoral artery, left femoral vein, and right radial artery, respectively. The activated clotting time was maintained between 250 and 300 seconds during the procedure. An 8Fr Emboguard Balloon Guide Catheter (Johnson & Johnson, New Brunswick, NJ, USA) was inserted through the 8Fr. Sheath and guided to the left cervical ICA for transarterial embolization. A 6Fr. guiding catheter BENCHMARK 071 (MEDICO’S HIRATA INC., Osaka, Japan) was inserted through the 6Fr. Sheath and guided transvenous to the left inferior petrosal sinus, followed by 2.4/1.7Fr. Microcatheter GREACH (Tokai Medical Products, Aichi, Japan) insertion into the BENCHMARK to access the shunted pouch with 0.014 inch microguidewire ASAHI CHIKAI (ASAHI INTECC, Aichi, Japan) using left carotid angiography as a reference. At this point, 3D rotational angiography (3DRA) of the left ICA was performed to determine the working angle, and it confirmed accurate guidance of GREACH to the shunted pouch [
A 4Fr. headhunter catheter was inserted through the 4Fr. Sheath for the right internal carotid angiography and vertebral angiography. Since the shunted flow is too fast to visualize the shunted pouch correctly with the conventional left internal carotid angiography, we performed the right internal carotid angiography, vertebral angiography, and left internal carotid angiography, respectively, under blocking of antegrade blood flow of the left ICA. The left internal carotid angiography, under blocking of the antegrade flow of itself was most useful to depict the accurate location of the shunted pouch. A 2.2/1.8Fr. microcatheter Phenom17 (Medtronic, Minneapolis, MN, USA) was also introduced into the BENCHMARK to reach the shunted point but was retained in the cavernous sinus due to interference at the shunt entrance with GREACH. SHOURYU 7 × 7 mm (Kaneka Medix, Osaka, Japan) balloon was inserted into the Emboguard and guided to the left cavernous ICA. A 3.4/3.2Fr. intermediate catheter TACTICS (Technocrat Corporation, Aichi, Japan) also was inserted into the Emboguard, and a 2.4/1.7Fr. Microcatheter SL-10 (Stryker, Kalamazoo, MI, USA) was inserted into the TACTICS. The SL-10 was guided transarterial to the shunted pouch with the ASAHI CHIKAI microguidewire. Under the balloon inflation of Emboguard and SHOURYU, a Target360 ULTRA 4 mm × 6 cm (Stryker) was inserted from the GREACH with balloon assist and detached. Subsequently, a HydroSoft 3D 3 mm × 6 cm (Terumo, Tokyo, Japan) and a HydroSoft 3D 2.5 mm × 4 cm (Terumo) were inserted from the SL-10 with balloon assist and the shunted flow disappeared [
Figure 3:
(a) The shunt obliterated after the 3rd coil insertion, although transvenous guided microcatheter (GREACH) remained in the shunted pouch, (b) GREACH was removed slightly out of the shunted pouch while leaving the microguidewire in the shunted pouch and the shunt was reignited (arrowheads), (c) GREACH was re-guided into the shunted pouch and the additional coil was inserted. GREACH was withdrawn spontaneously out of the shunted pouch and the shunt obliterated again.
Posttreatment course
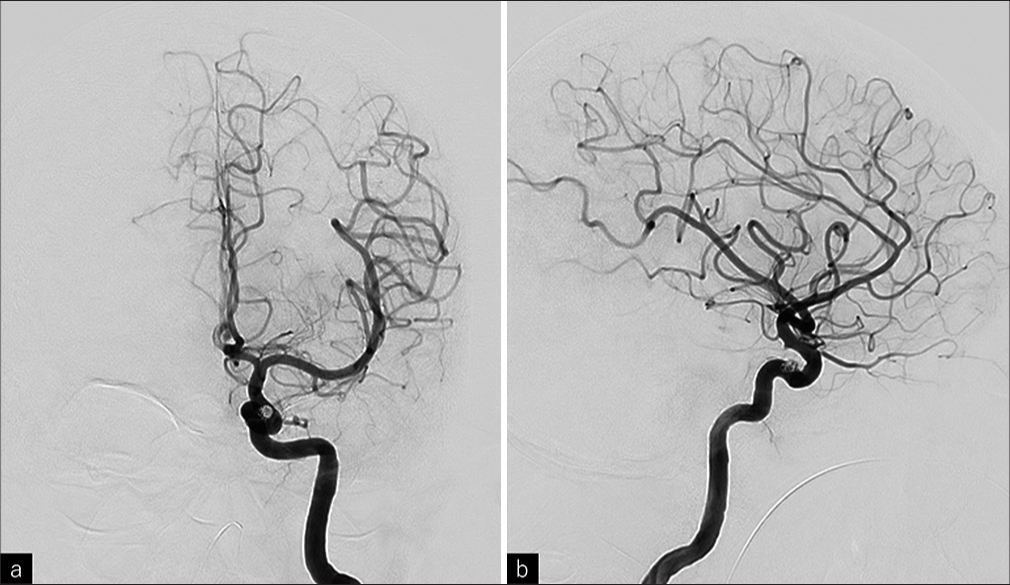
All clinical symptoms resolved immediately postoperatively. MRI on the following day showed a single hyperintensity spot in the left basal ganglia on diffusion-weighted imaging, though the patient remained asymptomatic. MRA confirmed no abnormal signals in the cavernous sinus. The patient was discharged on postoperative day 3 with a modified Rankin Scale score of 0, and during the 6-month follow-up period, there was no recurrence either clinically or on MRI imaging.
DISCUSSION
This case suggests that the rupture of a preexisting cavernous ICA aneurysm during cesarean delivery may have caused the direct CCF. However, in this case, there was no evidence of a preexisting aneurysm before the onset, so the cause cannot be determined. Another possible cause is the idiopathic dissection of the ICA, but it is more rare than aneurysm rupture. Since the cause cannot be determined, we referred to the pouch-like structure of the fistula as a “shunted pouch” in the text. It can be an aneurysm or the space in the cavernous sinus, which is divided by septa. While trauma is the most frequent cause of direct CCF,[
Historically, direct CCFs were treated with ICA ligation, muscle embolization, or radiotherapy, but these methods had low success and high complication rates.[
Although transvenous embolization is more commonly applied for indirect type CCFs (cavernous sinus dural arteriovenous fistula), it has also been applied in some direct CCF cases.[
There are a few reports of transvenous embolization as an additional treatment for residual lesions after transarterial embolization [
When the targeted embolization of the fistula is difficult, sinus packing of the cavernous sinus may be necessary. Cranial nerve palsy due to sinus packing of the cavernous sinus is reported to occur in 39.4% of cases.[
In this case, we thought that complete obliteration of the shunt could be achieved by targeted embolization of the shunted pouch, so we considered guiding multiple microcatheters into the shunted pouch to maximize the packing density of the shunted pouch. We decided to perform combined transarterial and transvenous embolization, considering the availability of a transarterial balloon for the sake of arterial flow control or protecting coil deviation to ICA and a smooth transition to transvenous sinus packing in case the targeted embolization does not successfully result in complete shunt obliteration.
Here, we outline four key points that were pivotal to the successful outcome.
The first one is about the identification of the shunt point. Since direct CCFs are characterized by high-flow shunt, it is often difficult to identify the accurate shunt point due to rapid blood flow, which can obscure imaging. The Huber maneuver (vertebral angiography performed under manual compression of the ipsilateral carotid artery) and the Mehringer–Hieshima maneuver (internal carotid angiography performed under manual compression of the proximal part of ipsilateral carotid artery) have been considered useful for identifying the shunt point.[
The second point is about reducing the amount of radiation exposure and contrast media used. We initially guided the microcatheter to the shunt pouch using only 2D imaging. A 3DRA scan was then obtained, which served two purposes: determining the optimal working angle and confirming the microcatheter’s precise positioning within the shunted pouch. We could have performed 3DRA before and after the guiding of the microcatheter, but the treatment of intracranial arteriovenous shunt diseases requires a large amount of radiation exposure and contrast media, and it is necessary for us to make efforts to reduce them as much as possible.
The third point is about the coil detachment method. Given the high flow shunt of direct CCFs, coil migration or distal embolization during the treatment may cause unexpected complications. We inflated the balloon of both an Emboguard balloon guide catheter and a SHOURYU2 assist balloon during coil embolization. After coil insertion, the SHOURYU2 balloon was deflated first, allowing blood flow through communicating arteries to confirm coil stability. Then, the Emboguard balloon was deflated while we monitored the coil’s position under antegrade flow in the left ICA, ensuring it remained in place before the final detachment. This multistep detachment approach minimized the risk of coil migration and embolic complications.
The fourth point is that after achieving shunt obliteration, we left a microguidewire in the shunt pouch while removing the microcatheter, as we anticipated the possibility of the shunt recurrence due to catheter displacement. The shunt actually recurred as we anticipated, but the guidewire facilitated rapid reaccess to the shunted pouch, allowing additional coil placement. After the additional coil placement, the shunt was completely obliterated again, and the microcatheter was kicked back out of the shunted pouch spontaneously. This strategic choice proved effective, although considering the possibility that the microcatheter cannot be redirected through the microguidewire into the shunted pouch, we should have continued coil embolization until the microcatheter spontaneously kicked back out of the shunted pouch even after the shunt disappeared. Since deviation of the coil loops from the shunted pouch is clinically acceptable on the venous side, in the final stage of treatment, transvenous embolization is preferable, as was done in this case.
CONCLUSION
We successfully achieved targeted embolization of the shunted pouch in a case of direct CCF using a limited number of coils by combined transarterial and transvenous embolization. This case demonstrates practical strategies and considerations for achieving complete shunt obliteration with minimal intervention.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985. 62: 248-56
2. Chi CT, Nguyen D, Duc VT, Chau HH, Son VT. Direct traumatic carotid cavernous fistula: Angiographic classification and treatment strategies. Study of 172 cases. Interv Neuroradiol. 2014. 20: 461-75
3. Debrun G, Lacour P, Caron JP, Hurth M, Comoy J, Keravel Y. Detachable balloon and calibrated-leak balloon techniques in the treatment of cerebral vascular lesions. J Neurosurg. 1978. 49: 635-49
4. Fukawa N, Nakagawa N, Tsuji K, Yoshioka H, Furukawa K, Nagatsuka K. Transarterial and transvenous coil embolization of direct carotid-cavernous fistulas. J Neuroendovasc Ther. 2022. 16: 127-34
5. Hamano E, Satow T, Hori T, Takahashi JC, Kataoka H. A case of direct carotid-cavernous fistulae successfully treated by bidirectional double catheter technique: A technical note. J Neuroendovasc Ther. 2022. 16: 307-12
6. Henderson AD, Miller NR. Carotid-cavernous fistula: Current concepts in aetiology, investigation, and management. Eye (Basingstoke). 2018. 32: 164-7
7. Hoffman H, Ashok Kumar A, Wood JS, Mikhailova T, Yoo JH, Wakeman MB. Outcomes after endovascular treatment of direct carotid cavernous fistulas: Systematic review and meta-analysis. World Neurosurg. 2023. 170: e242-55
8. Huber P. A technical contribution of the exact angiographic localization of carotid cavernous fistulas. Neuroradiology. 1976. 10: 239-41
9. Ide S, Kiyosue H, Tokuyama K, Hori Y, Sagara Y, Kubo T. Direct carotid cavernous fistulas. J Neuroendovasc Ther. 2020. 14: 583-92
10. Kohli GS, Patel BC, editors. Carotid cavernous fistula. StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. p.
11. Lv X, Jiang C, Li Y, Yang X, Wu Z. Recovery of opthalmoplegia associated with cavernous sinus dural arteriovenous fistulas after transvenous cavernous sinus packing. Eur J Radiol. 2010. 75: 139-42
12. Mehringer CM, Hieshima GB, Grinnell VS, Tsai F, Pribram HF. Improved localization of carotid cavernous fistula during angiography. AJNR Am J Neuroradiol. 1982. 3: 82-4
13. Nishino K, Ito Y, Hasegawa H, Kikuchi B, Shimbo J, Kitazawa K. Cranial nerve palsy following transvenous embolization for a cavernous sinus dural arteriovenous fistula: Association with the volume and location of detachable coils. J Neurosurg. 2008. 109: 208-14
14. Prasad SN, Singh V, Boruah DK, Phadke RV, Sharma K, Kannaujia V. Endovascular management of direct carotid-cavernous fistula: Evolution of cost effective sandwich technique. J Neurosci Rural Pract. 2020. 11: 558-64
15. Ringer AJ, Salud L, Tomsick TA. Carotid cavernous fistulas: Anatomy, classification, and treatment. Neurosurg Clin N Am. 2005. 16: 279-95
16. Viñuela F, Fox AJ, Debrun GM, Peerless SJ, Drake CG. Spontaneous carotid-cavernous fistulas: Clinical, radiological, and therapeutic considerations. Experience with 20 cases. J Neurosurg. 1984. 60: 976-84