- Department of Neurosurgery, Tokyo Metropolitan Neurological Hospital, Fuchu, Japan
- Department of Neurology, Tokyo Metropolitan Neurological Hospital, Fuchu, Japan
Correspondence Address:
Takeshi Matsuo, Department of Neurosurgery, Tokyo Metropolitan Neurological Hospital, Fuchu, Japan.
DOI:10.25259/SNI_931_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryo Onoda1, So Fujimoto1, Kota Bokuda2, Toshio Shimizu2, Takeshi Matsuo1. Discrepancy between preoperative noninvasive evaluation and intraoperative electrical cortical stimulation for motor function assessment in diffuse cortical dysplasia. 06-Jun-2025;16:231
How to cite this URL: Ryo Onoda1, So Fujimoto1, Kota Bokuda2, Toshio Shimizu2, Takeshi Matsuo1. Discrepancy between preoperative noninvasive evaluation and intraoperative electrical cortical stimulation for motor function assessment in diffuse cortical dysplasia. 06-Jun-2025;16:231. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13608
Abstract
Background: Evaluating residual motor function and functional compensation is essential before performing hemispherotomy. We have been evaluating hand motor function using transcranial magnetic stimulation (TMS), which cannot confirm the lower limb functions.
Case Description: A male teenager with a huge arachnoid cyst in the right frontotemporal region and extensive polymicrogyria and gyral dysplasia in the adjacent lobes experienced focal to bilateral tonic-clonic seizures. We performed functional magnetic resonance imaging (MRI) and electrical cortical stimulation in addition to TMS. Functional MRI and TMS-motor-evoked potential (MEP) results suggested that the left primary motor cortex elicited the bilateral motor response, while intraoperative cortical stimulation MEPs revealed that the primary motor areas of each lower limb were controlled contralaterally. Consequently, we performed a total callosotomy instead of a hemispherotomy.
Conclusion: The results suggest that a preoperative diagnosis of complete hemispheric damage based on noninvasive examinations is not sufficient in some cases to determine the operative strategy. A combination of pre- and intraoperative examinations may be required to prevent unexpected neurological complications.
Keywords: Functional magnetic resonance imaging, Hemispherotomy, Intraoperative electrical cortical stimulation, Total corpus callosotomy, Transcranial magnetic stimulation
INTRODUCTION
Evaluation of the residual motor function of the affected cerebral hemisphere and functional compensation of the unaffected hemisphere is essential before performing hemispherotomy. Transcranial magnetic stimulation (TMS) and functional magnetic resonance imaging (fMRI) are often used in addition to structural magnetic resonance imaging (MRI). Herein, we report a case of a patient with intractable epilepsy with unilateral diffuse cortical dysplasia, in whom there was a discrepancy between TMS, fMRI, and intraoperative electrical cortical stimulation responses.
CASE DESCRIPTION
The patient was a male teenager who had no visible abnormalities at birth but had mild mental retardation. Febrile seizures were observed at 3 and 7 years of age, but neither was of the complex type. He had no family history of central nervous system disorders such as epilepsy. At 6 months, diffuse cortical dysplasia of the right cerebral hemisphere was detected through a detailed examination for slight movement disorders of the left upper and lower limbs. Antiseizure therapy was initiated when the patient first experienced focal to bilateral tonic-clonic seizures at 14 years of age. Because the seizure suppression was limited and generalized convulsions persisted, the patient was referred to our hospital for surgical treatment 2 years after disease onset. At the first visit, he was alert and conscious. He had mild left hemiparesis with predominance in the upper extremities, poor finger dexterity, and poor pronation and supination of the upper extremities. Although he was able to walk, his left lower limb dragged slightly when he ran. He had focal impaired awareness seizures that occurred weekly and focal-onset bilateral tonic-clonic seizures that occurred monthly. According to the Wechsler Adult Intelligence Scale-Fourth Edition, his full-scale intelligence quotient was 62, which was in the mild impairment range.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Examination
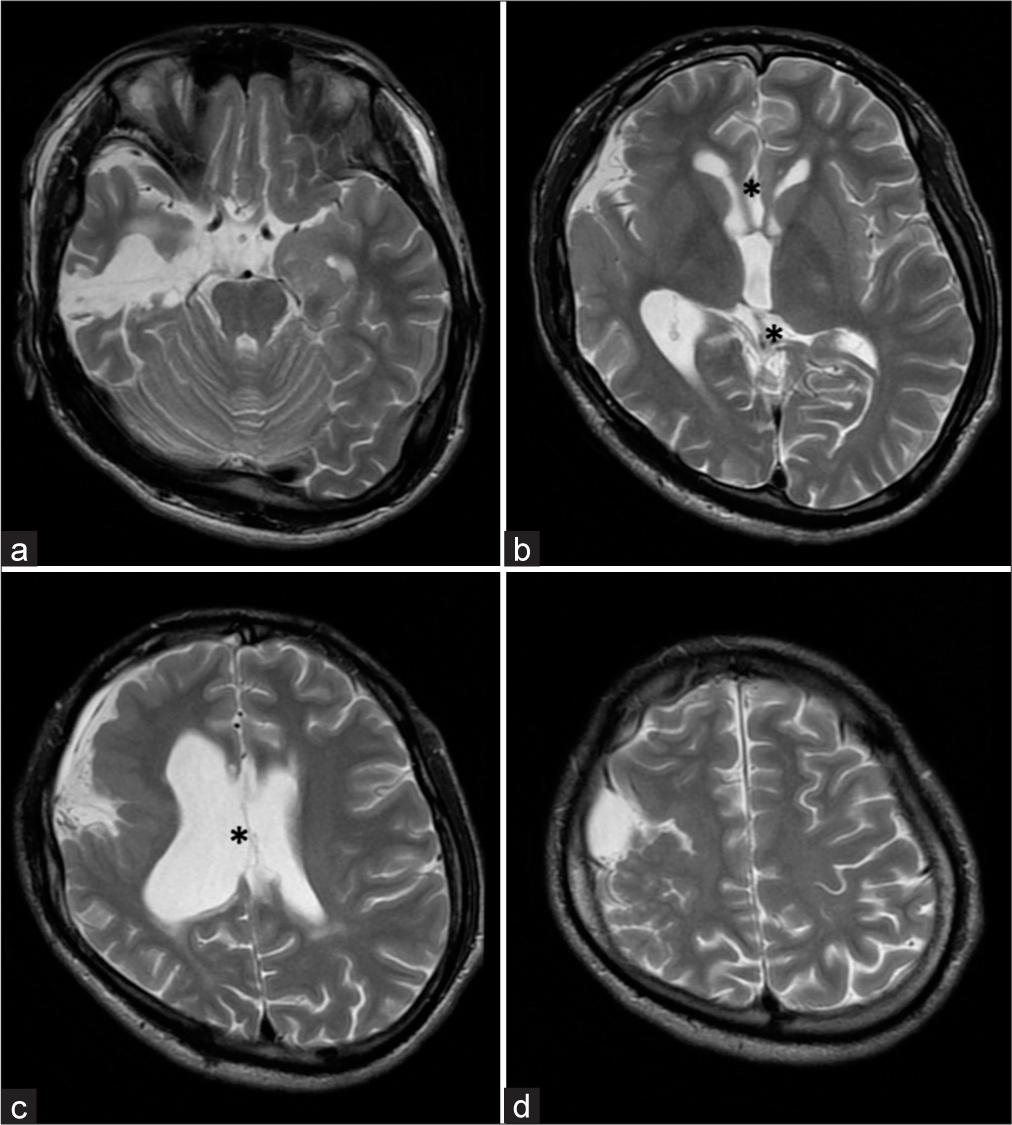
Interictal electroencephalography (EEG) showed rhythmic slow waves, intermittent sharp waves, and spiked slow waves from the right posterior temporal region to the occipital region at a frequency of 1/1 min. The ictal onset on EEG was in the right centroparietal region, with symptoms of deviation of the eyes and head to the left and clonic posturing of the bilateral upper limbs. MRI revealed an arachnoid cyst measuring 8 × 4 cm in maximum diameter in the right frontotemporal region, and extensive polymicrogyria and gyral dysplasia in the adjacent frontal lobe, parietal lobe, insular gyrus, and temporal lobe. Secondary atrophy of the right thalamus, cerebral peduncle, pons, and medulla oblongata was also observed. In addition, T2-weighted images revealed signal attenuation and obscurity in the right pyramidal tract [
Figure 1:
Preoperative structural magnetic resonance images. (a-d) Short-T1 inversion recovery (STIR) images revealed an arachnoid cyst in the right front-temporal region (arrows). (b-d) STIR images also revealed polymicrogyria and dysplasia of the gyri in the right frontal, temporal, parietal and insular lobes (arrow heads) adjoining the arachnoid cyst. Conversely, the right occipital lobe and gyri in the interhemispheric region appeared morphologically normal.
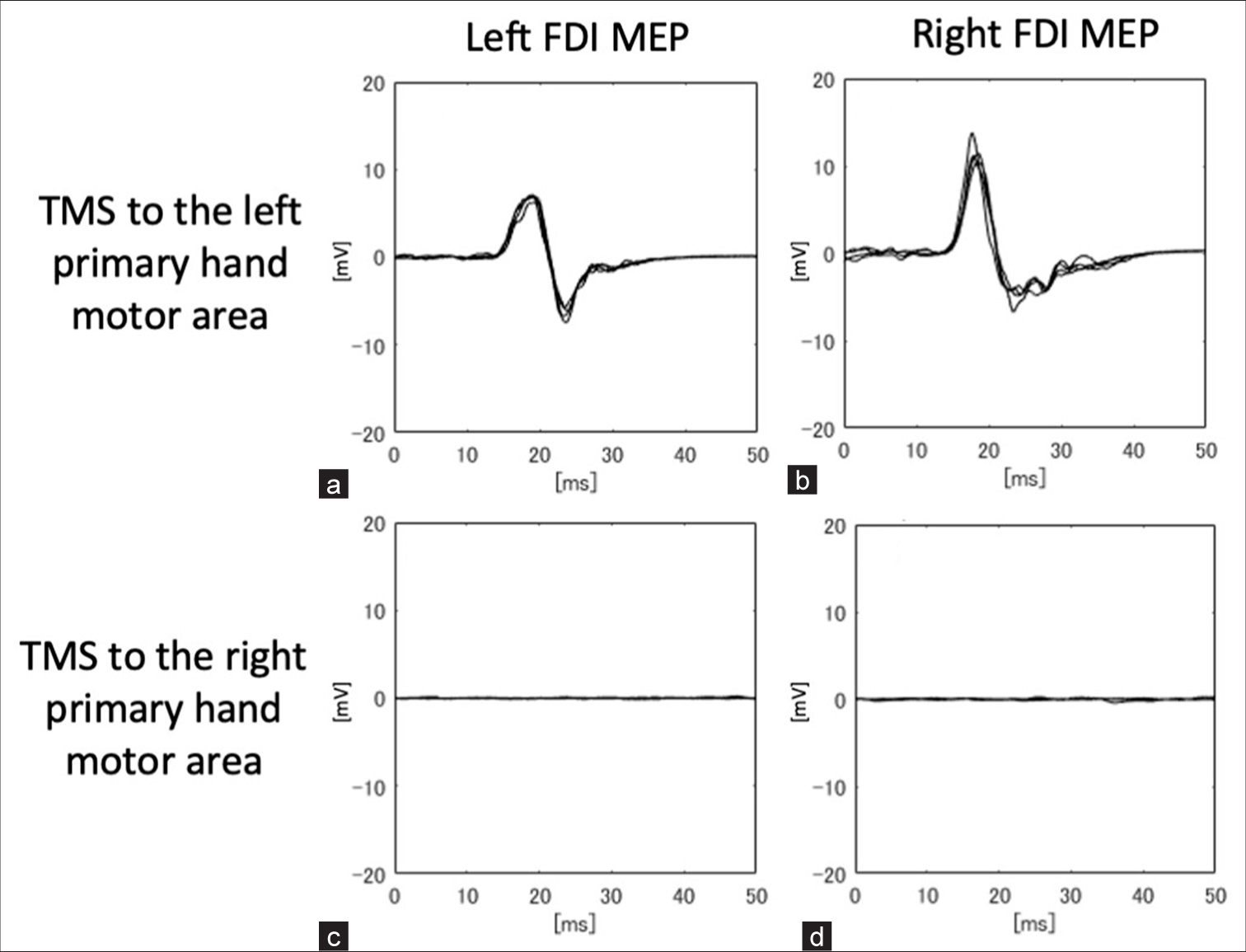
TMS of the left primary hand motor area evoked bilateral motor-evoked potentials (MEPs) in the first dorsal interosseous (FDI) muscles and the biceps brachii muscles during maximal voluntary contraction of each muscle (The Magstim Company Ltd., Whitland, UK; figure-of-eight coil, 80% stimulus intensity). There were no differences in latency between the left and right muscles. The MEP latencies for the left and right FDIs were 21.7 and 21.8 ms, respectively. Similarly, we searched for and stimulated an area thought to be the right primary motor cortex, but no MEP was evoked from muscles of either side (80–100% stimulus intensity) [
Figure 2:
Results of transcranial magnetic stimulation-motor-evoked potentials (TMS-MEPs). (a and b) TMS of the left primary hand motor area evoked potentials in the left first dorsal interosseous (FDI) muscle and right FDI. The MEP latencies of the left and right FDI were 21.7 and 21.8 ms, respectively, during the preoperative examination. (c and d) No motor response was elicited from either the left FDI or the right FDI when the right motor cortex was stimulated.
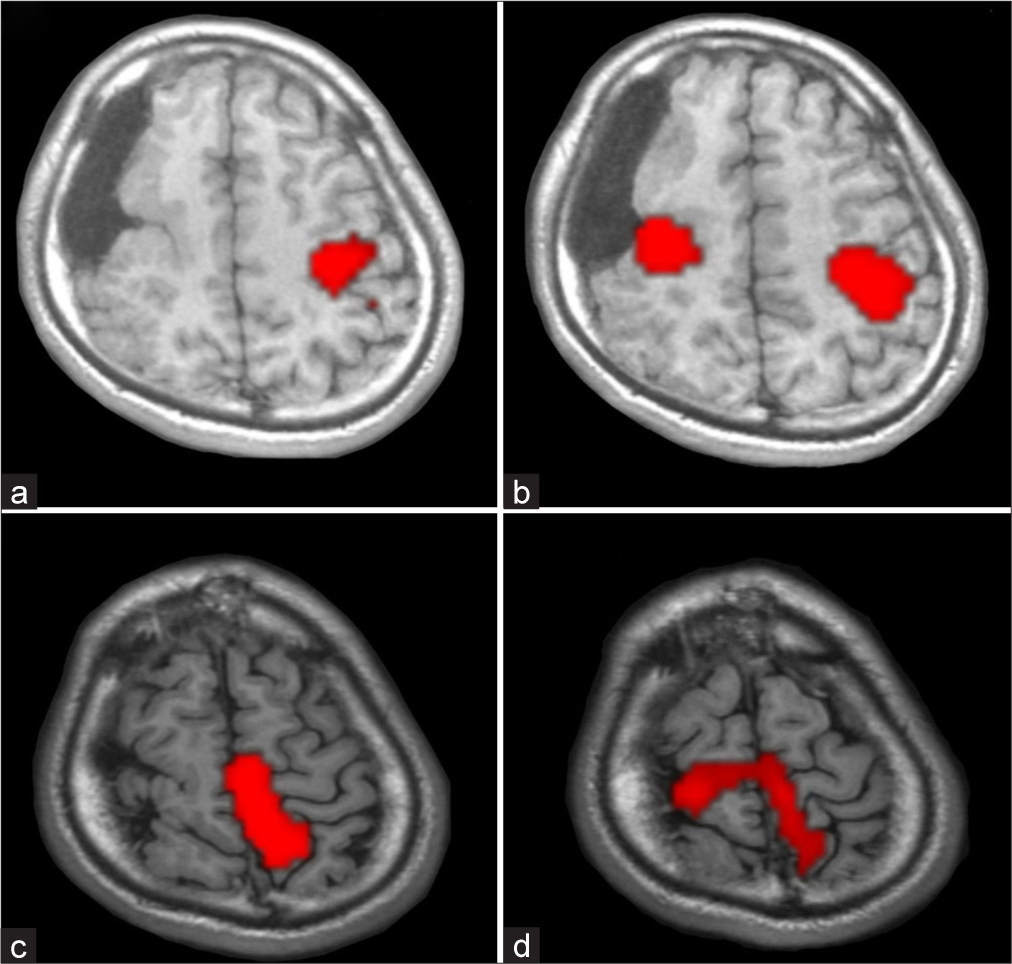
fMRIs were acquired using a 3.0 Tesla MRI (Discovery MR750, GE HealthCare, Chicago, IL, USA). The functional scan had the following parameters: repetition time/echo time, 3000/30 ms; flip angle, 80°; field of view, 220 mm; and slice thickness, 3.2 mm. The fMRI paradigms were implemented using block designs (block length: 30 s) with alternating periods of silent rest and activation. Each scan consisted of six blocks. A task of right finger tapping induced activation of the left precentral gyrus, and a task of left finger tapping induced activation of the bilateral precentral gyrus. In addition, extension and flexion of the right ankle activated the left precentral gyrus in the interhemispheric fissure, whereas extension and flexion of the left ankle activated the bilateral precentral gyri in the interhemispheric fissure [
Figure 3:
Activation patterns of functional magnetic resonance imaging. (a) Right finger tapping activated the left precentral gyrus. (b) Left finger tapping activated the bilateral precentral gyri. (c) Right ankle transitive motion activated the left precentral gyrus on the interhemispheric fissure. (d) Left ankle transitive motion activated the bilateral precentral gyri on the interhemispheric fissure.
In the Wada test, propofol was injected at 1 mg/s from the terminal end of the left internal carotid artery, and language impairment and right upper limb paresis occurred after 15 mg was injected. When propofol was injected on the right side, mild motor weakness of the left upper limb was observed after 10 mg was injected.
Visual field testing revealed left homonymous hemianopia.
Clinical course
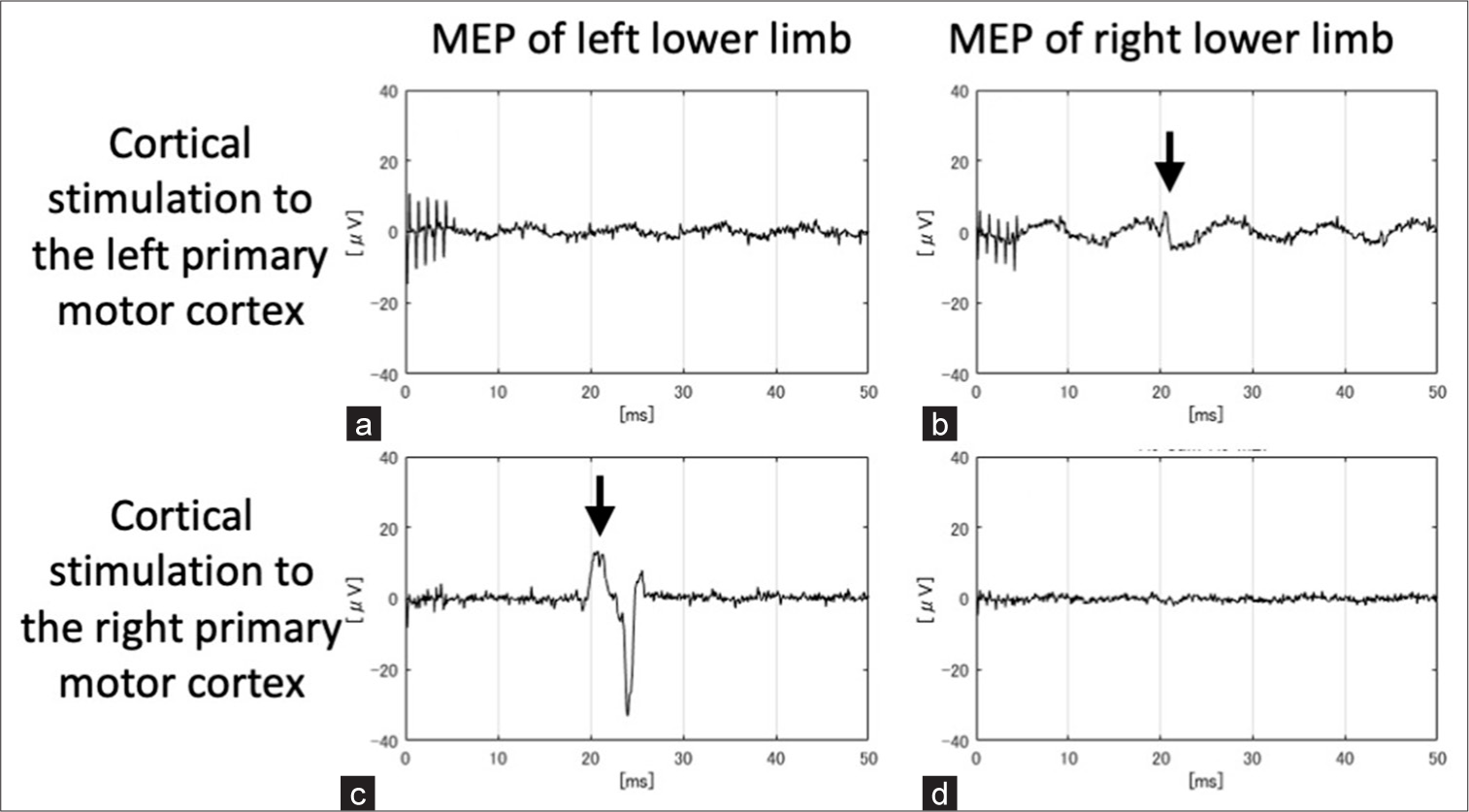
Based on the results of the preoperative fMRI and TMS, we determined that the unaffected left primary motor cortex controlled bilateral limb movements, and we decided to perform a right hemispherotomy with a vertical approach. Right frontal craniotomy was performed, and silicon-lined platinum electrodes (inter-electrode distance, 10 mm; diameter, 3 mm) were inserted back-to-back into the interhemispheric fissure facing the lower limb primary motor cortex. Electrical stimulation of those areas was performed separately with a monophasic positive pattern (duration 0.5 ms, interval 2.0 ms, and 5 pulses). MEPs were elicited only in the right lower limb at 20 mA when the left primary motor cortex was stimulated, whereas they were elicited only in the left lower limb at 15 mA when the right primary motor cortex was stimulated. The MEP latencies of the right and left lower limbs were 41.0 and 41.3 ms, respectively [
Figure 4:
Results of intraoperative cortical motor-evoked potentials (MEPs). (a) MEPs were not detected in the left lower limb when the left primary motor cortex was stimulated. (b) MEPs were elicited in the right lower limb when the left primary motor cortex was stimulated (arrow). (c) MEPs were elicited in the left lower limb when the right primary motor cortex was stimulated (arrow). (d) MEPs were not detected in the right lower limb when the right primary motor cortex was stimulated. (b and c) The MEP latencies of the right and left lower limbs were 41.0 and 41.3 ms, respectively.
DISCUSSION
Hemispherotomy requires that the healthy cerebral hemisphere completely or largely compensate for brain function on the affected side. The indications for hemispherotomy are comprehensively determined based on clinical symptoms, structural/functional imaging, and functional evaluation of the motor cortex.
Whether a motor function of the affected cerebral hemisphere remains or is impaired is evaluated using TMS and fMRI. In patients with extensive lesions in one cerebral hemisphere from the congenital or early birth stage, MEPs are not elicited by TMS of the affected hemisphere but are recorded in the ipsilateral distal muscles with TMS of the unaffected hemisphere. These patient groups exhibit no complications even after hemispherotomy.[
In this patient, using intraoperative electrical cortical stimulation, we determined that the affected hemisphere controlled the contralateral lower limb. The surgical procedure was changed from hemispherotomy to corpus callosotomy, which might have prevented paralysis of the left lower limb. If the patient were an infant, we would have anticipated that he would recover through plasticity, even if severe lower-limb paralysis had appeared after hemispherotomy.
This study is based on a single case report, which limits the generalizability of our findings. Our results suggest the potential limitations of preoperative functional assessments and individual anatomical variations and may influence the discrepancies observed between preoperative and intraoperative evaluations.
CONCLUSION
In brain surgery for patients with brain malformations, it is essential to determine the surgical procedure based on the results of preoperative noninvasive examinations. However, flexibility is also required to change the surgical procedure during surgery, depending on the results of intraoperative electrical stimulation. This case highlights the potential limitations of preoperative functional assessments and emphasizes the importance of intraoperative motor function evaluation when discrepancies arise between pre- and intraoperative findings.
Ethical approval:
The research/study approved by the Institutional Review Board at Tokyo Metropolitan Neurologcal Hospital, number TS-R06-009, dated May 28, 2024.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship:
This work was supported by the MHLW Research program on rare and intractable diseases, Grant number JPMH23FC1013.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment:
This work was supported by the MHLW Research program on rare and intractable diseases, Grant number JPMH23FC1013.
References
1. Kamida T, Fujiki M, Abe T, Baba H, Kobayashi H. Application of transcranial magnetic MEP to epilepsy surgery: The decision of operative course and the relationship with seizure outcome. No Shinkei Geka. 2005. 33: 1151-9
2. Sasaki N. The efficacy of rTMS for stroke in the early phase. Jpn J Rehabil Med. 2019. 56: 28-32
3. Shimizu T, Nariai T, Maehara T, Hino T, Komori T, Shimizu H. Enhanced motor cortical excitability in the unaffected hemisphere after hemispherectomy. Neuroreport. 2000. 11: 3077-84
4. Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: A TMS and fMRI study. Brain. 2002. 125: 2222-37
5. Staudt M, Krägeloh-Mann I, Holthausen H, Gerloff C, Grodd W. Searching for motor functions in dysgenic cortex: A clinical transcranial magnetic stimulation and functional magnetic resonance imaging study. J Neurosurg. 2004. 101: 69-77
6. Zsoter A, Pieper T, Kudernatsch M, Staudt M. Predicting hand function after hemispherotomy: TMS versus fMRI in hemispheric polymicrogyria. Epilepsia. 2012. 53: e98-101