- Department of Neurosurgery, Kumamoto University Hospital, Kumamoto, Japan
- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- Department of Neurosurgery, Oita University Faculty of Medicine, Yufu, Japan
- Department of Neurosurgery, Kyushu Medical Center, Fukuoka, Japan
- Department of Diagnostic Pathology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan
- Department of Laboratory Medicine, National Cancer Center Hospital, Chuo-ku, Japan
- Department of Neurosurgery, Kurume University School of Medicine, Kurume, Japan
Correspondence Address:
Akitake Mukasa, Department of Neurosurgery, Kumamoto University Hospital, Kumamoto, Japan.
DOI:10.25259/SNI_734_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hirotaka Inoue1, Jun-Ichiro Kuroda1, Yutaka Fujioka2, Nobuhiro Hata3, Masahiro Mizoguchi4, Daiki Yoshii5, Hiroyuki Sueyoshi1, Yuki Takeshima1, Kenji Fujimoto1, Naoki Shinojima1, Kuniko Sunami6, Yoshiki Mikami5, Hideo Nakamura7, Akitake Mukasa1. Drug-resistant BRAF V600E-mutant recurrent pleomorphic xanthoastrocytoma, CNS WHO Grade 3 successfully resolved with incidental discontinuation of combined BRAF and MEK inhibitor therapy. 15-Nov-2024;15:417
How to cite this URL: Hirotaka Inoue1, Jun-Ichiro Kuroda1, Yutaka Fujioka2, Nobuhiro Hata3, Masahiro Mizoguchi4, Daiki Yoshii5, Hiroyuki Sueyoshi1, Yuki Takeshima1, Kenji Fujimoto1, Naoki Shinojima1, Kuniko Sunami6, Yoshiki Mikami5, Hideo Nakamura7, Akitake Mukasa1. Drug-resistant BRAF V600E-mutant recurrent pleomorphic xanthoastrocytoma, CNS WHO Grade 3 successfully resolved with incidental discontinuation of combined BRAF and MEK inhibitor therapy. 15-Nov-2024;15:417. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13228
Abstract
Background: Combination therapy with BRAF and MEK inhibitor holds promise for treating gliomas harboring the BRAF V600E mutation; however, the development of acquired resistance remains a challenge.
Case Description: We describe a case of repeated recurrent BRAF-mutant pleomorphic xanthoastrocytoma (central nervous system World Health Organization grade 3) treated with combination therapy with BRAF and MEK inhibitor. The patient received dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor); however, she developed resistance to the combination therapy. Remarkably, incidental drug discontinuation contributed to the disappearance of the resistant tumor. The same phenomenon was repeatedly observed after that. Genetic analysis demonstrated that the resistant tumor had BRAF V600E amplification; the resistant tumor remained BRAF→MEK→ERK pathway dependent, and drug resistance might be due to elevated BRAF V600E expression. We speculated that ERK1/2 signal extremes caused by the discontinuation of the combination therapy affected the resistant tumor survival.
Conclusion: This case study provides important insights into novel treatment strategies and their underlying mechanisms for gliomas with BRAF mutations.
Keywords: Anaplastic pleomorphic xanthoastrocytoma, BRAF, Dabrafenib, Drug holiday, Trametinib
INTRODUCTION
Combination therapy with BRAF and MEK inhibitor shows promise for treating BRAF V600E mutation-harboring gliomas, yet developing acquired resistance remains challenging. The median response duration to the combination therapy for central nervous system (CNS) World Health Organization (WHO) grade 3 pleomorphic xanthoastrocytoma (PXA) is 6 months due to drug resistance.[
CASE PRESENTATION
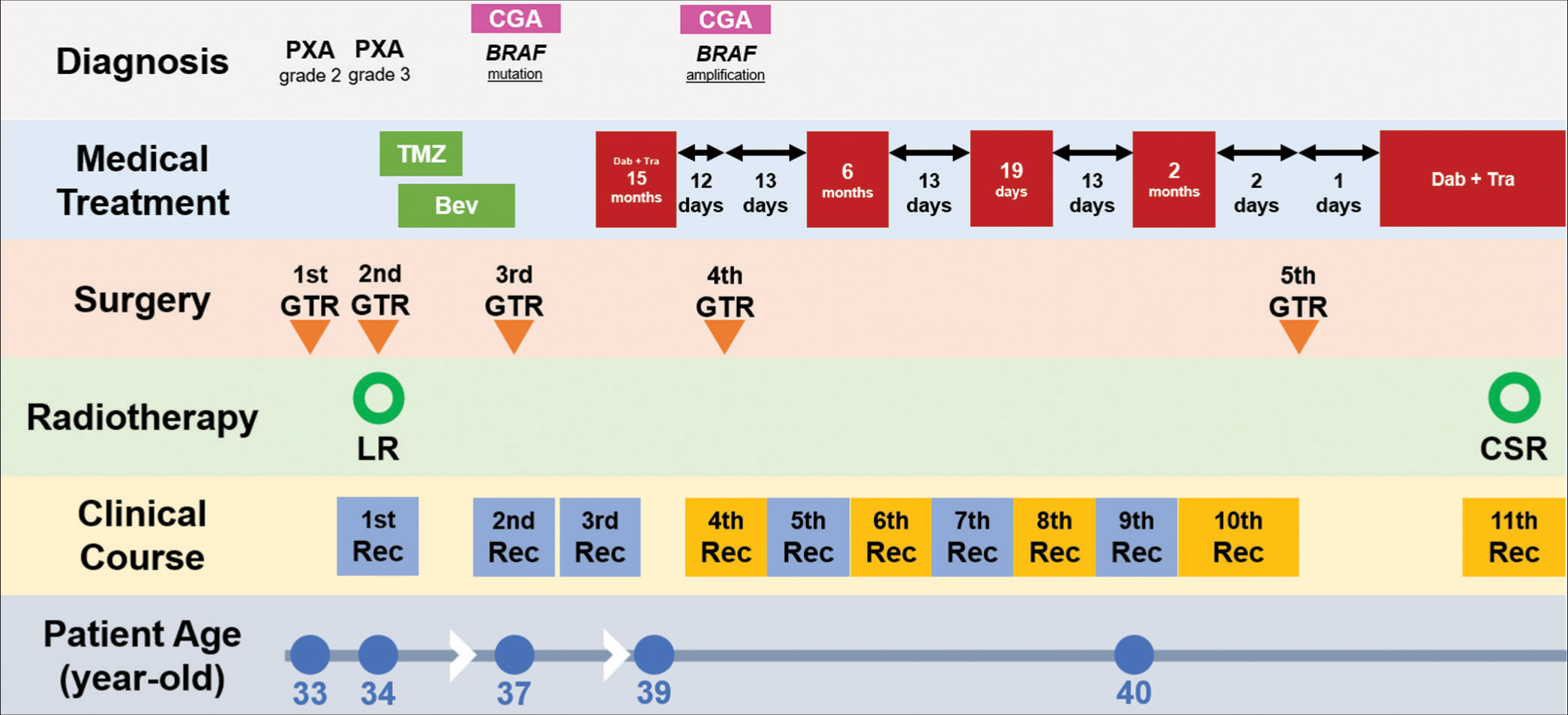
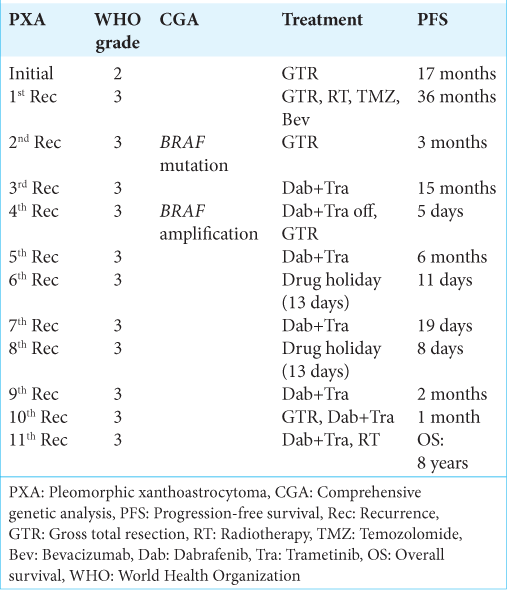
Here, we describe a female patient with CNS WHO grade 3 PXA who experienced repeated recurrences of resistance to combination therapy with BRAF and MEK inhibitors. The patient’s clinical course and management timeline are shown in
Figure 1:
Treatment Timeline. Timeline summarizing the treatment course of our patient, including surgery, chemotherapy, radiation therapy, and molecular-targeted therapy. The red box indicates dabrafenib + trametinib; gaps in the red boxes indicate drug holidays. The yellow boxes indicate resistant tumors. PXA: Pleomorphic xanthoastrocytoma, CGA: Comprehensive genetic analysis, Rec: Recurrence, GTR: Gross total resection, TMZ: Temozolomide, Bev: Bevacizumab, LR:Local radiation, CSR: Craniospinal radiation.
The recurrent tumor harbored a BRAF V600E mutation, and the patient participated in a pan-cancer multidrug off-label treatment trial, BELIEVE (NCCH1901, jRCTs031190104).[
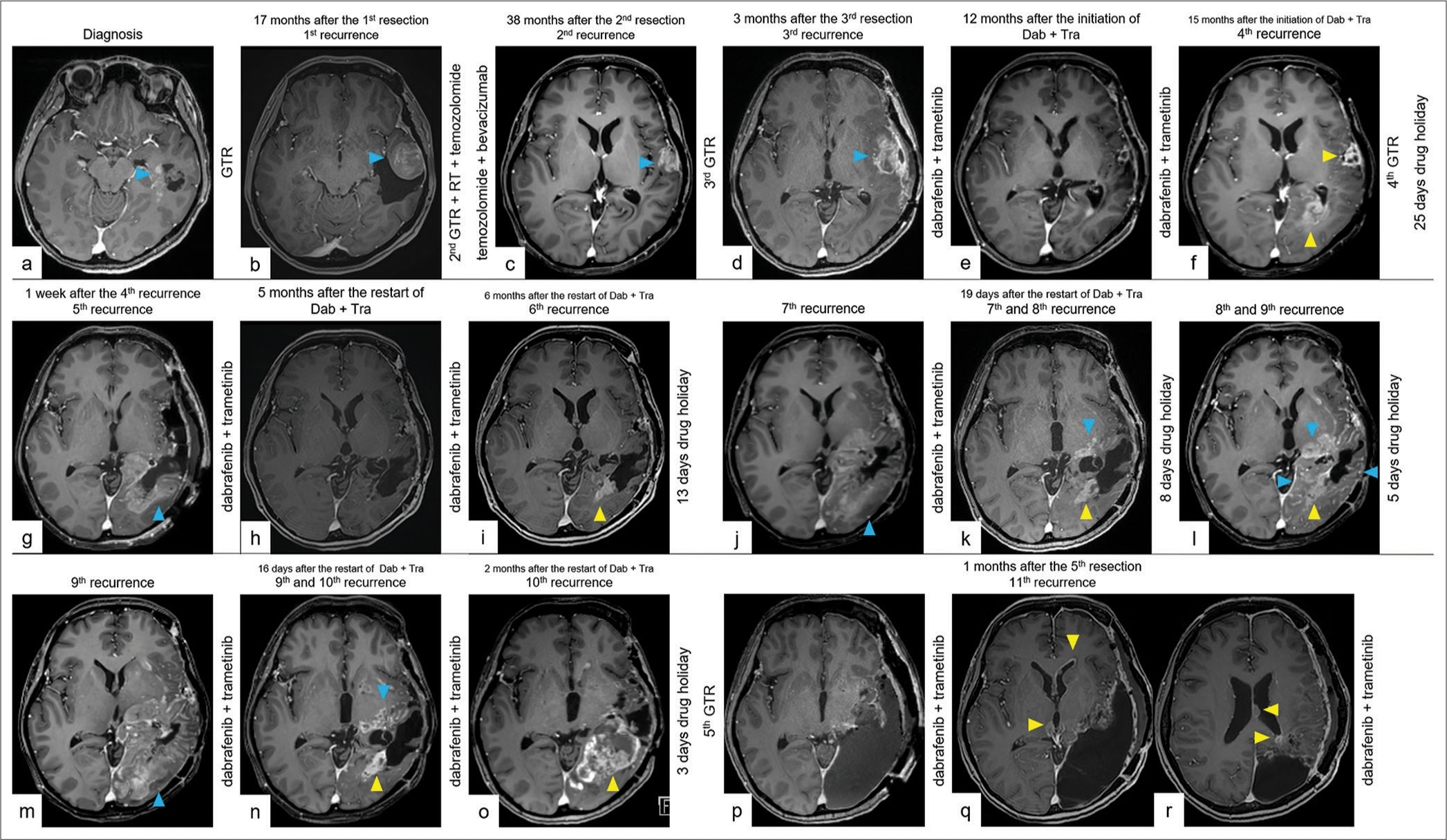
A subsequent restart of the combination therapy was effective for 6 months, but the drug-resistant tumor reappeared at the atrium [
Figure 2:
The key imaging course of drug holiday. (a) CET1WI on MRI showed the sixth recurrence. (b) CET1WI on MRI showed the seventh recurrence with the disappearance of the sixth recurrence. (c) CET1WI on MRI showed the eighth recurrence with regression of the seventh recurrence due to restarting the combination therapy. (d and e) CET1WI on MRI showed the ninth recurrence with gradual dissipation of the eighth recurrence during drug holiday. (f) CET1WI on MRI showed the tenth recurrence with dissipation of the ninth recurrence. (g) CET1WI on MRI showed the tenth recurrence. (h) CET1WI on MRI after the salvage surgery. The yellow arrowhead indicates a recurrent tumor that acquired resistance to combination therapy with BRAF and MEK inhibitors. The blue arrowhead indicates a drug-sensitive tumor. CET1WI: Contrast-enhanced T1-weighted imaging, MRI: magnetic resonance imaging.
After the creatine kinase level improved, the combination therapy was once again effective against the diffuse tumor [
Figure 3:
The full imaging course with repeated recurrences. (a) CET1WI on MRI showed a 2.6 cm cystic tumor (blue arrowhead) in the left temporal lobe at the time of diagnosis. (b) CET1WI on follow-up MRI 17 months after the first resection showing the first local recurrent tumor (blue arrowhead). (c) CET1WI on follow-up MRI 38 months after the second resection showing the second local recurrent tumor (blue arrowhead) after GTR of the first recurrent tumor, radiotherapy, temozolomide, and bevacizumab. (d) CET1WI on follow-up MRI 3 months after the third resection showing the third local recurrent tumor (blue arrowhead) after GTR of the second recurrent tumor. Dab + Tra was initiated 2 months after the diagnosis of the third recurrence. (e) CET1WI on follow-up MRI 12 months after the initiation of Dab + Tra, showing tumor regression. (f) CET1WI on follow-up MRI 15 months after the initiation of Dab + Tra showing the fourth local recurrence-resistant tumor (yellow arrowheads). Dab + Tra was then discontinued. (g) CET1WI on follow-up MRI 1 week after the fourth recurrence showing the fifth diffuse recurrent tumor (blue arrowhead) after GTR of the fourth recurrent tumor. Dab + Tra was restarted 25 days after the drug holiday. (h) CET1WI on follow-up MRI 5 months after restarting Dab + Tra showing stable disease. (i) CET1WI on follow-up MRI 6 months after restarting Dab + Tra, showing the sixth local recurrent-resistant tumor (yellow arrowhead). Dab + Tra was then discontinued. (j) CET1WI on follow-up MRI after 10 days of drug holiday showing the seventh diffuse recurrent tumor (blue arrowhead) with the disappearance of the sixth recurrent tumor. Dab + Tra was resumed 13 days after the drug holiday. (k) CET1WI on follow-up MRI 19 days after the second restart of Dab + Tra showing the eighth local recurrent-resistant tumor (yellow arrowhead), with a reduction in the contrast effect of the seventh tumor (blue arrowhead). Dab + Tra was then discontinued. (l) CET1WI on follow-up MRI after 7 days of drug holiday showing the ninth diffuse recurrent tumor (blue arrowheads) with gradual dissipation of the contrast effect of the eighth recurrent tumor (yellow arrowheads). (m) CET1WI on follow-up MRI after 12 days of drug holiday showing the ninth diffuse recurrent tumor (blue arrowhead) with the disappearance of the eighth recurrent tumor. Dab + Tra was resumed 13 days after the drug holiday. (n) CET1WI on follow-up MRI 16 days after the third restart of Dab + Tra showing the tenth local recurrent-resistant tumor (yellow arrowhead), with a reduction in the contrast effect of the ninth tumor (blue arrowhead). Dab + Tra was then administered. (o) CET1WI on follow-up MRI 2 months after the third restart of Dab + Tra showing the growing tenth recurrent-resistant tumor (yellow arrowhead) and an intratumoral hemorrhage. (p) CET1WI on follow-up MRI a day after the fifth resection. Dab + Tra was restarted on the same day. (q) CET1WI on follow-up MRI a month after the fifth resection, showing the eleventh disseminated recurrence in the ventricular wall (yellow arrowheads). (r) CET1WI on follow-up MRI a month after the fifth resection, showing the eleventh disseminated recurrence around the resection cavity and in the ventricular wall (yellow arrowheads). CET1WI: Contrast-enhanced T1-weighted imaging, MRI: Magnetic resonance imaging, GTR: Gross total resection, RT: radiotherapy, Dab: Dabrafenib, Tra: Trametinib.
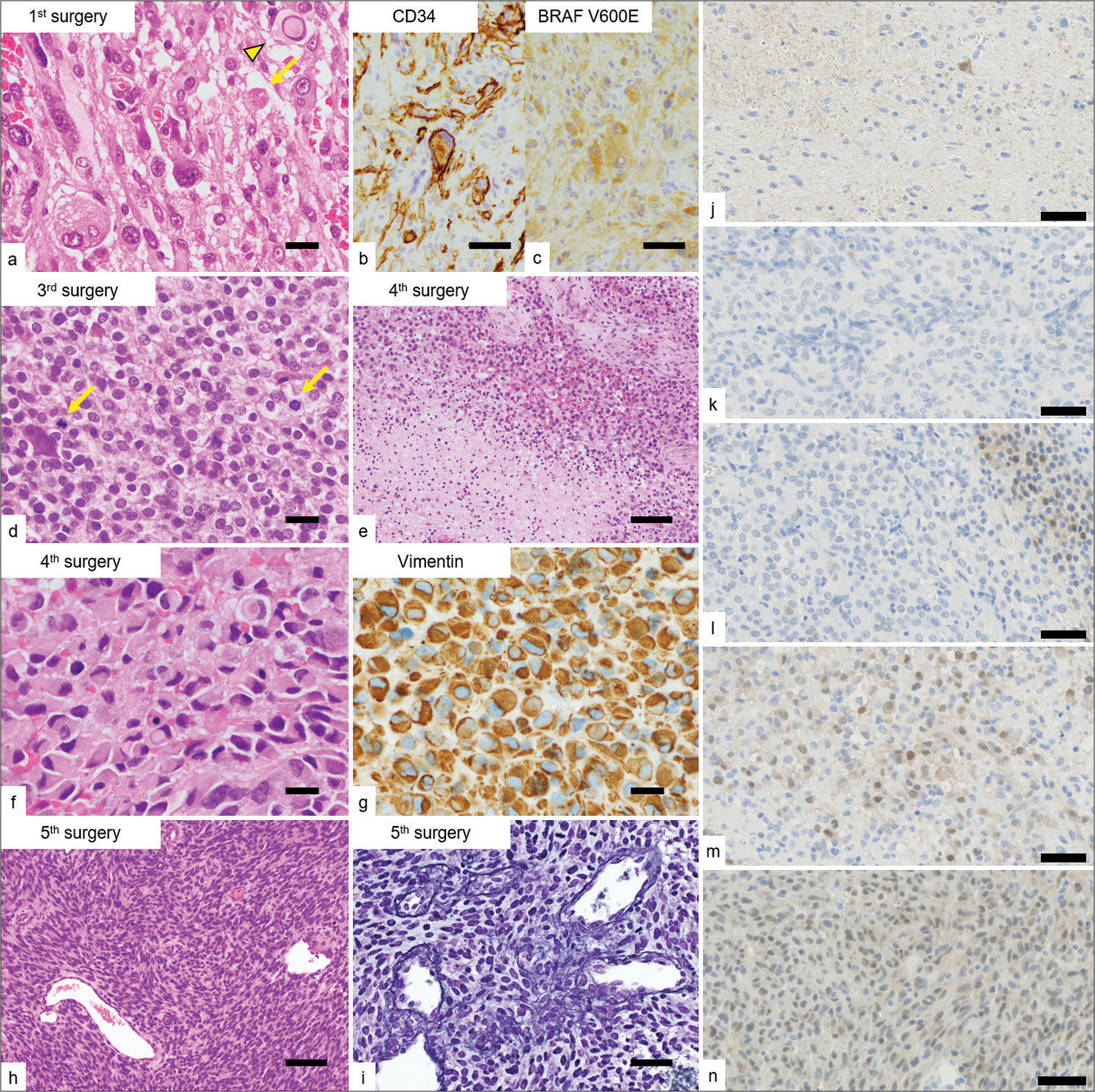
Figure 4:
Microscopic appearances of the resected specimens. (a) The proliferation of pleomorphic and spindle cells with occasional intranuclear pseudo-inclusions (arrowhead) and eosinophilic granular bodies (arrow) in the initially resected specimen. (b and c) Immunohistochemically, the tumor cells were positive for CD34 and BRAF V600E-mutant proteins. (d) The proliferation of tumor cells with epithelioid features showing brisk mitotic activity (arrows) in the recurrent tumor (3rd resection). (e) Coagulative tumor necrosis in the recurrent tumors (4th resection). (f and g) The proliferation of rhabdoid cells with vimentin-positive cytoplasmic inclusions in the recurrent tumors (4th resection). (h) The proliferation of spindle cells in an interlacing fascicular pattern with occasional intervening reticulin fibers in the recurrent tumor, imparts a sarcomatoid appearance (5th resection). (i) Reticulin staining (5th resection). (j-n) ERK1/2 staining (each resection). The ERK1/2 signaling of drug-resistant tumors was expressed strongly despite the presence of the combination therapy with BRAF and MEK inhibitor. (m and n) Scale bars (a, d, f, g) 20 µm, (b, c, i, j, k, l, m, n) 50 µm, and (e, h) 100 µm.
DISCUSSION
The most remarkable aspect of this case study was that incidental drug discontinuation contributed to the control of the resistant tumor repeatedly.
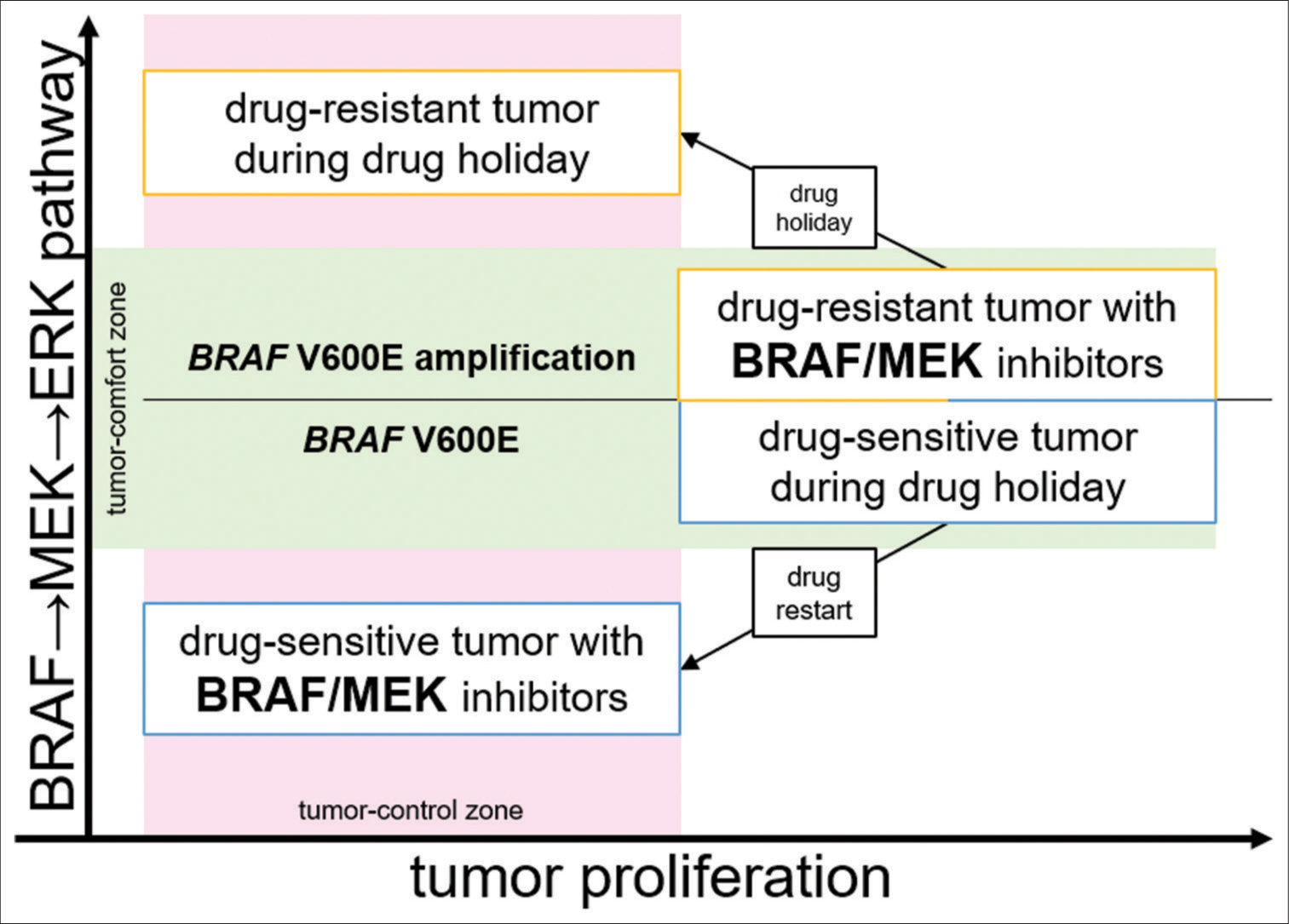
A preclinical model demonstrated that resistant melanomas become drug dependent for proliferation and regress when BRAF inhibitor is attenuated. ERK1/2 signal, which is downstream of BRAF, operates within a tightly defined fitness threshold to drive tumor proliferation. Drug discontinuation causes ERK1/2 signal extremes, leading to cell cycle arrest or apoptosis.[
In our patient, drug-resistant tumors were controlled only by incidental drug holidays, although for a limited time. As this is a single case report, any discussion of the mechanism of the clinical course is speculative; however, preclinical studies seemed to reflect our patient’s clinical course.[
On the other hand, ERK1/2 staining showed increased positivity in resistant tumors [
The preclinical study showed drug discontinuation with upregulated BRAFy, supported this hypothesis ds) containing specifiulting in cell cycle arrest or apoptosis.[
CONCLUSION
Herein, we report the case of a patient with CNS WHO grade 3 PXA who developed resistance to combination therapy with BRAF and MEK inhibitors. Incidental drug discontinuation contributed to the control of the resistant tumor. This case study provides important insights into novel treatment strategies and their underlying mechanisms for gliomas with BRAF mutations. Owing to the rarity of this tumor and the paucity of previous reports, the unique clinical course of our patient is worth reporting. Further molecular studies may help to understand whether the appropriate administration method of molecularly targeted therapy may delay the acquired resistance or control tumors.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Algazi AP, Othus M, Daud AI, Lo RS, Mehnert JM, Truong TG. Continuous versus intermittent BRAF and MEK inhibition in patients with BRAF-mutated melanoma: A randomized phase 2 trial. Nat Med. 2020. 26: 1564-8
2. Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. 2010. 3: ra84
3. Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013. 494: 251-5
4. Ishimaru S, Shimoi T, Sunami K, Nakajima M, Ando Y, Okita N. Platform trial for off-label oncology drugs using comprehensive genomic profiling under the universal public healthcare system: The BELIEVE trial. Int J Clin Oncol. 2024. 29: 89-95
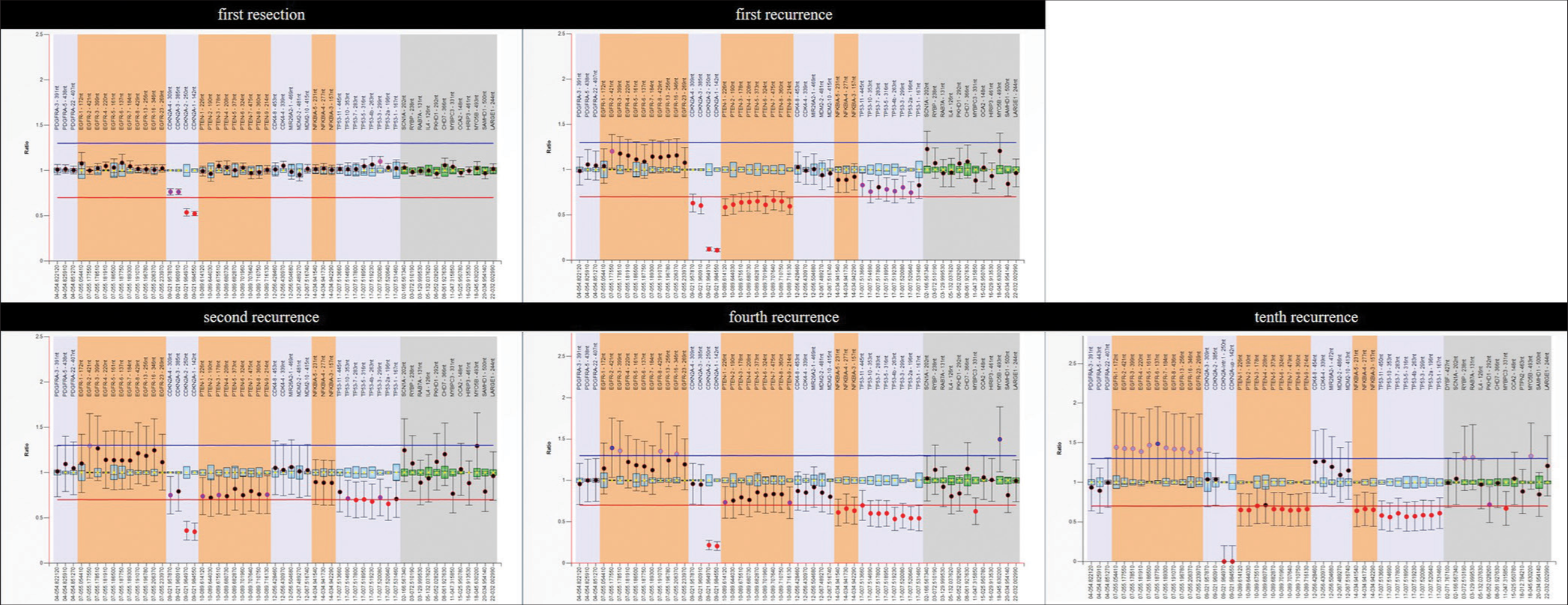
5. Neilsen BK, Sleightholm R, McComb R, Ramkissoon SH, Ross JS, Corona RJ. Comprehensive genetic alteration profiling in primary and recurrent glioblastoma. J Neurooncol. 2019. 142: 111-8
6. Sasame J, Ikegaya N, Kawazu M, Natsumeda M, Hayashi T, Isoda M. HSP90 inhibition overcomes resistance to molecular targeted therapy in BRAFV600E-mutant high-grade glioma. Clin Cancer Res. 2022. 28: 2425-39
7. Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FY. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): A multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022. 23: 53-64