- Department of Anatomy, Kirksville College of Osteopathic Medicine, Kirksville, Missouri, United States.
Correspondence Address:
Bruce A. Young, Department of Anatomy, Kirksville College of Osteopathic Medicine, Kirksville, Missouri, United States.
DOI:10.25259/SNI_365_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Connor J. English, Zachary Taylor, Michael Cramberg, Bruce A. Young. Dynamic asymmetry in cerebrospinal fluid pressure: An indicator of regional differences in compliance. 02-Jun-2023;14:187
How to cite this URL: Connor J. English, Zachary Taylor, Michael Cramberg, Bruce A. Young. Dynamic asymmetry in cerebrospinal fluid pressure: An indicator of regional differences in compliance. 02-Jun-2023;14:187. Available from: https://surgicalneurologyint.com/surgicalint-articles/12349/
Abstract
Background: Dural compliance influences the shape and magnitude of the cerebrospinal fluid (CSF) pulsations. In humans, cranial compliance is approximately 2× greater than spinal compliance; the differential has been attributed to the associated vasculature. In alligators, the spinal cord is surrounded by a large venous sinus, which suggests that the spinal compartment may have higher compliance than is found in mammals.
Methods: Pressure catheters were surgically implanted into the cranial and spinal subdural spaces of eight subadult American alligators (Alligator mississippiensis). The CSF was propelled through the subdural space by orthostatic gradients and rapid changes in linear acceleration.
Results: CSF pressure recordings taken from the cranial compartment were consistently, and significantly, larger than those taken from the spinal compartment. After the myodural bridge of Alligator was surgically released, the asymmetry in CSF pressure was decreased.
Conclusion: Unlike the situation in humans, the spinal compartment of Alligator has greater compliance than the cranial compartment, presumably due to the presence of the large spinal venous sinus surrounding the dura. The change in CSF pressures after myodural surgical release supports the hypothesis that the myodural bridge functions, at least in part, to modulate dural compliance and the exchange of CSF between the cranial and spinal compartments.
Keywords: Cerebrospinal fluid, Compliance, Meninges, Myodural bridge, Pressure propagation
INTRODUCTION
Recordings of resting, or anesthetized, human cerebrospinal fluid (CSF) pressures yield a distinctive series of pulsations, driven by the cardiac and ventilatory cycles.[
The compliance in the mammalian central nervous system (CNS) is not constant. In the skull, the dura is fused to the periosteum, effectively making much of the epidural space a potential one,[
The present study was conducted on the American alligator (Alligator mississippiensis). In the alligator, the spinal cord extends throughout the trunk and the tail,[
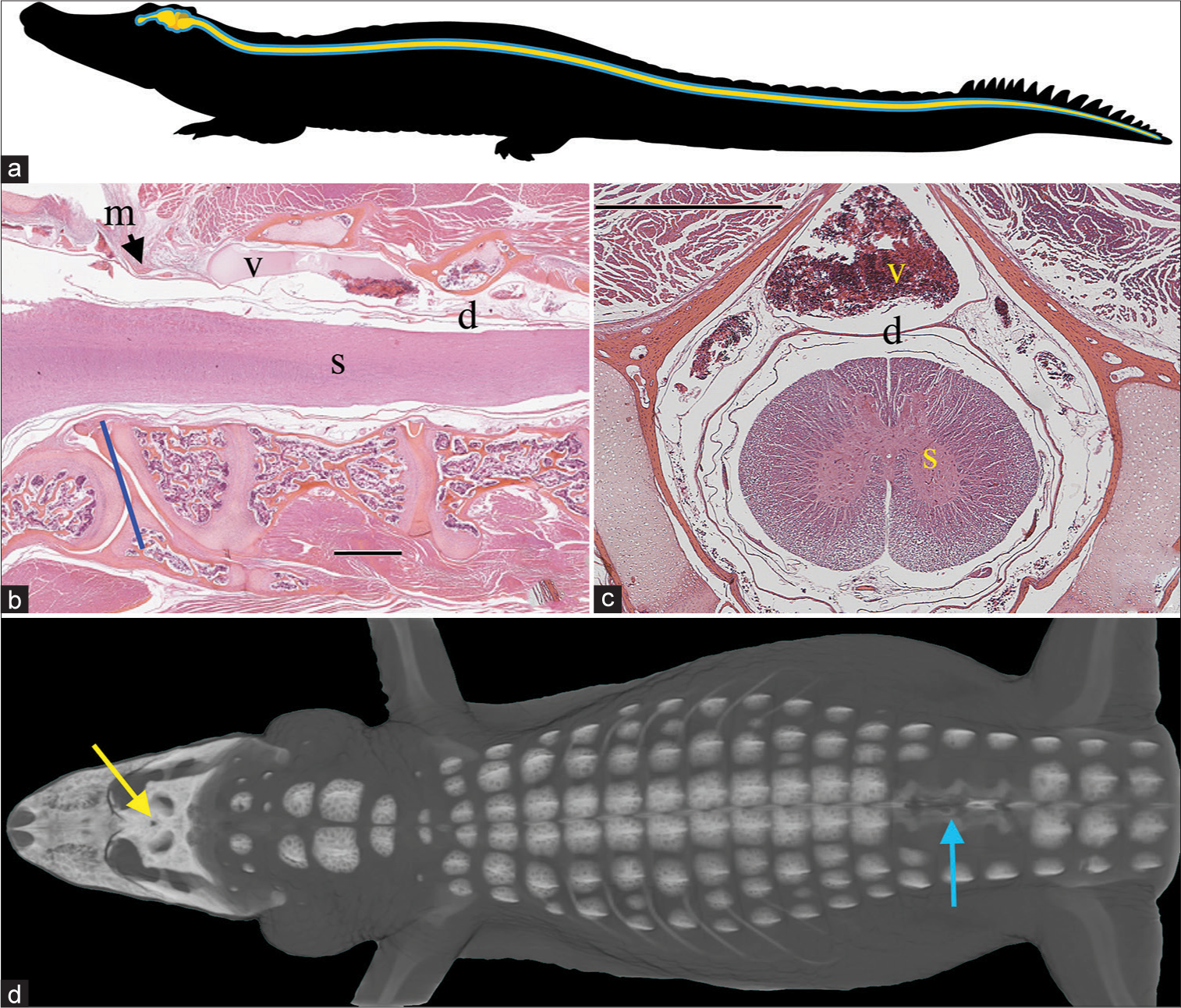
Figure 1:
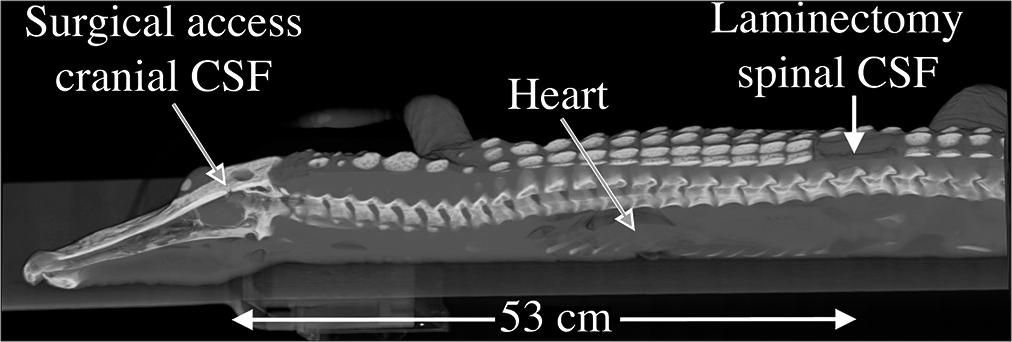
Spinal anatomy of Alligator mississippiensis. (a) Schematic of the central nervous system (yellow) and surrounding cerebrospinal fluid (CSF)-filled subdural space (blue); the spinal cord extends to the tip of the tail. (b) Sagittal section through the foramen magnum (blue line) showing the spinal cord (s), dura (d), and the surrounding spinal venous sinus (v). As the dura transitions from the cranial to spinal configuration, it is contacted by the skeletal muscle fibers of the myodural bridge (m). The black line is cited as the scale bar (c) Transverse section through the cervical spinal region showing the spinal venous sinus (v) around the dura (d) and spinal cord (s). (d) Surgical approach illustrated on a 3-D reconstruction of CT data from a 172 cm alligator. The small surgical opening in the skull (yellow arrow) was used to record the cranial CSF pressure, while a laminectomy was performed (blue arrow) to allow for recordings of spinal venous blood pressure and spinal CSF pressure. Scale bars in b and c are both 1 mm.
The purpose of this study was to examine how the compliance of the dural complex of Alligator, particularly the presence of the surrounding venous blood-filled spinal sinus and the myodural bridge, influenced pressure pulsations in the CSF. Previous studies have explored the functional “exchange” of CSF between the cranial and spinal compartments.[
MATERIALS AND METHODS
Live animals
Eight subadult (150–187 cm total length) A. mississippiensis were obtained from the Louisiana Department of Wildlife and Fisheries. The alligators were housed communally in a 29 m2 facility that featured three submerging ponds, natural light, and artificial lights on a 12:12 cycle. The facility was maintained at 30–33°C, warm water rain showers were provided every 20 min, which helped maintain the facility at >75% relative humidity. The alligators were maintained on a diet of previously frozen adult rats. The husbandry and use of the live alligators followed all applicable federal guidelines and were approved by the IACUC of A.T. Still University (Protocol #226, approved March 2022).
Basic experimental preparation
When an individual animal was removed from the enclosure, it was caught by noosing, and its jaws taped shut around a bite pad using vinyl tape. The alligator was then placed on a stiff board (244 × 28 × 3.8 cm thick), which exceeded the maximum width and length of the alligators used for this study. Six 2.5 cm wide heavy duty straps (Northwest Tarp and Canvas; Bellingham, WA) were used to secure the alligator to the board; the straps were tight enough to minimize movement of the animal but not tight enough to impede ventilation or circulation.
With the alligator’s mouth held open by the bite pad, a laryngoscope was used to depress the gular valve and expose the glottis. A cuffed endotracheal tube was inserted into the larynx and connected to a custom anesthesia system that included a ventilator pump (Harvard), Vaporstick anesthesia machine (Surgivet), isoflurane vaporizer (Surgivet), and Capnomac Ultima respiratory gas monitor (Datex-Engstrom). The alligators were maintained on a steady ventilatory pattern of 6–8 breaths/min (depending on size) each with a tidal volume of 500 mL. Anesthesia was accomplished using 5% isoflurane. The alligator’s EKGs were recorded using two silver chloride surface cup electrodes (019-477200, GRASS, Natus Medical, Pleasanton, CA), coated with a layer of conducting gel (Signagel, Parker Laboratories, Fairfield, NJ), and placed on the ventral surface of the animal on either side of the heart. The electrodes were connected to a P511 preamplifier (GRASS).
A stainless steel surgical burr was used to bore an approximately 4 mm diameter portal through the sagittal midline of the skull just caudal to the orbits. This allowed for direct exposure of the dura mater; a small incision in the dura was used to inset a segment of polyethylene (PE) tubing into the subarachnoid space. A laminectomy was performed at the equivalent of the L2 level to expose the venous sinus [
The pressure catheters were connected to P23AA fluid pressure transducers (Statham), which were filled with reptilian Ringer’s solution. The pressure transducers were mounted to the board at a fixed site immediately adjacent to, and level with, the surgical exposures, so that rotation of the alligator did not produce a pressure head between the PE tubing and the transducer. The implantation of the PE tubing was snug enough that no CSF leakage was observed; yet, the functionality of the coupling was evident by the (pressure-driven) movement of the CSF along a distance of the catheter. The pressure transducers were coupled to P122 preamplifiers (GRASS). The cranial and spinal pressure catheters were separated by a mean of 51.6 cm. This experimental preparation resulted in stable baseline CSF pressure waves that reflected a combination of ventilatory (at ~0.18 Hz) and cardiac (at ~0.33 Hz) influences [
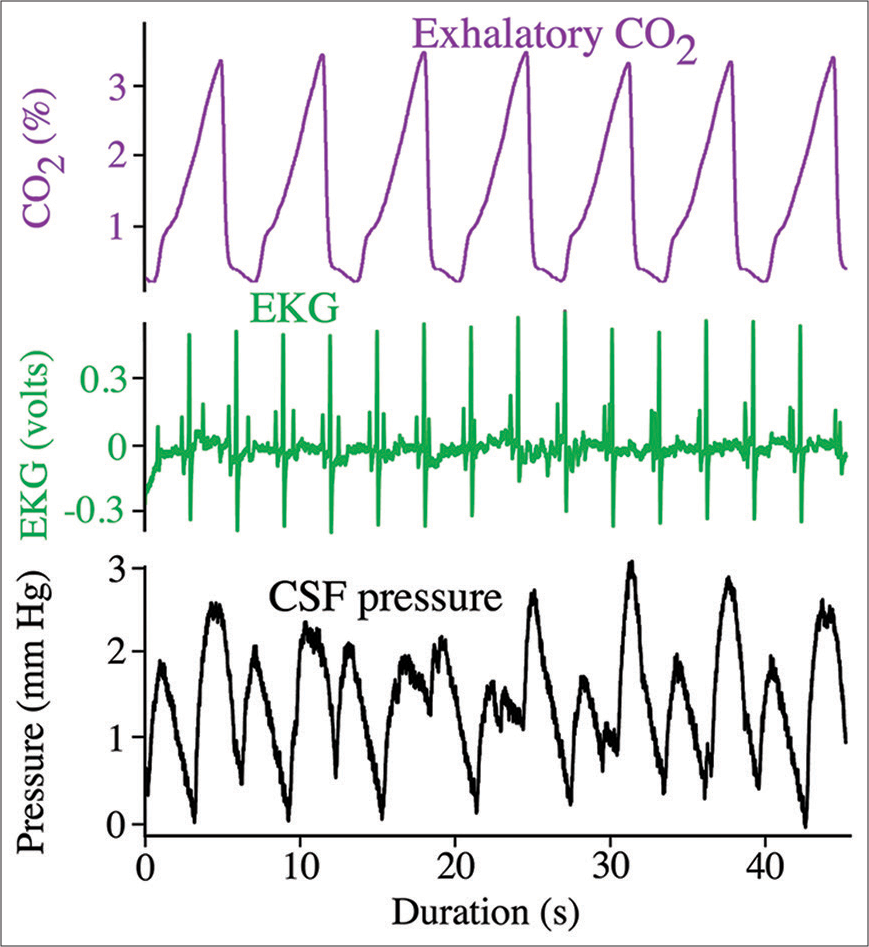
Figure 2:
Simultaneous recordings of the ventilatory cycle (top trace, purple), cardiac cycle (middle trace, green), and cranial cerebrospinal fluid (CSF) pressure (bottom trace, black) from Alligator mississippiensis. Note that the CSF pressure contains pulsations linked to both the cardiac and ventilatory cycles.
The CSF pressures, EKG, and ventilatory pattern (from the exhalatory gas analyzer) were recorded simultaneously (at 4.0 kHz) using the MiDas data acquisition system (Xcitex). The CSF pressure transducers were individually calibrated following each experiment. The recorded signals were quantified using the MiDas software, then exported for power spectral analysis using SpectraPlus (Pioneer Hill Software).
Propelling the CSF
Once a stable surgical baseline had been established, two different methods were used to generate experimental pulses in the CSF. After the implantation of the spinal venous or CSF pressure catheter, the alligator was exposed to an orthostatic gradient by rotating the preparation 30° head-up or head-down. The axis of rotation was located approximately 3 cm caudal to the spinal pressure catheter; in all preparations, the cranial and spinal pressure catheters were on the same side of the pivot point for rotation.
Following the orthostatic trials, the surgical board the alligator was strapped to was placed onto a cart with casters resting in a track. The transducers were anchored to the surgical board, so their position relative to the alligator or the pressure catheter did not change. The pressure catheter itself was buttressed between the surgical incision and the transducer so that it was not impinged on, but all displacement was prevented. The cart supporting the anesthetized alligator was then quickly accelerated forward or backward over a distance of approximately 30 cm, then abruptly stopped. The acceleration and stopping was performed manually, this, combined with occasional contact between the casters and the track, meant that the push-pull curves were not always perfectly smooth [
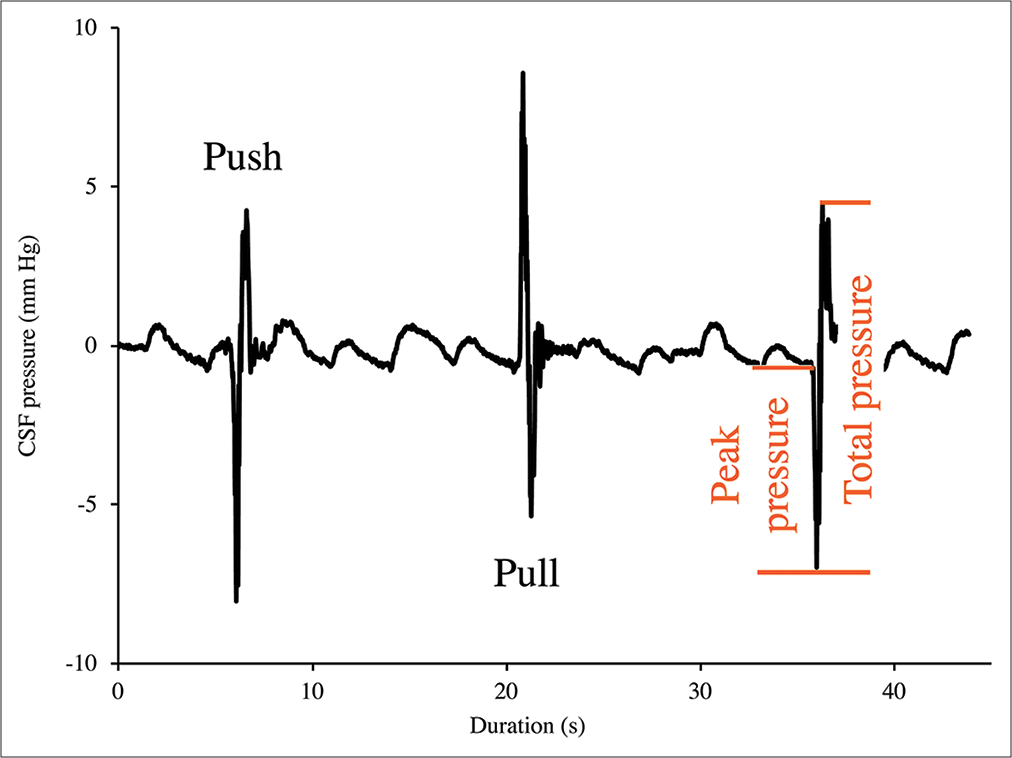
Figure 3:
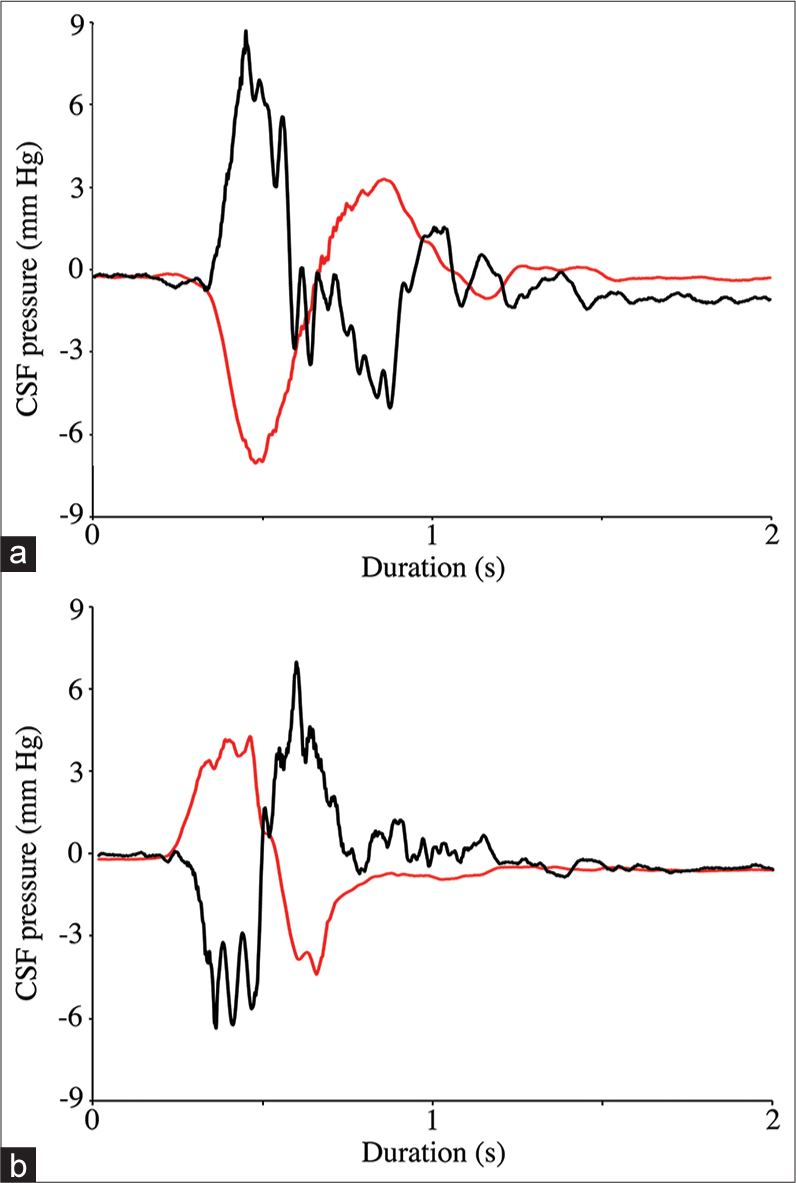
Cranial cerebrospinal fluid (CSF) pressure from three consecutive “push-pull” trials. The push trials result in an initial decrease in cranial CSF pressure, followed by a larger pressure increase; the pull trials result in an initial increase in cranial CSF pressure followed by a larger pressure decrease. For all the trials, the amplitude of the initial spike was defined as “peak pressure” while the amplitude of the following (larger) spike was defined as “total pressure.”
The displacements of the alligator were recorded using digital video cameras (Action Camera, YI Technology). The visual records were imported into Kinovea (kinovea.org), the instantaneous velocities were determined and combined with the mass of the alligator to yield momentum.
Myodural release
After a full set of data were collected, a surgical myodural release was performed. The goal of this release was to disrupt all the tendinous attachments to the dura on both the rostral and caudal sides of the atlas, without disrupting the structural integrity of the spinal dura itself. An additional round of push/ pull trials was performed following the myodural release.
RESULTS
Orthostatic gradients
The orthostatic gradients produced consistent results, in part, because there was no evidence of a compensatory mechanism. Three key results [
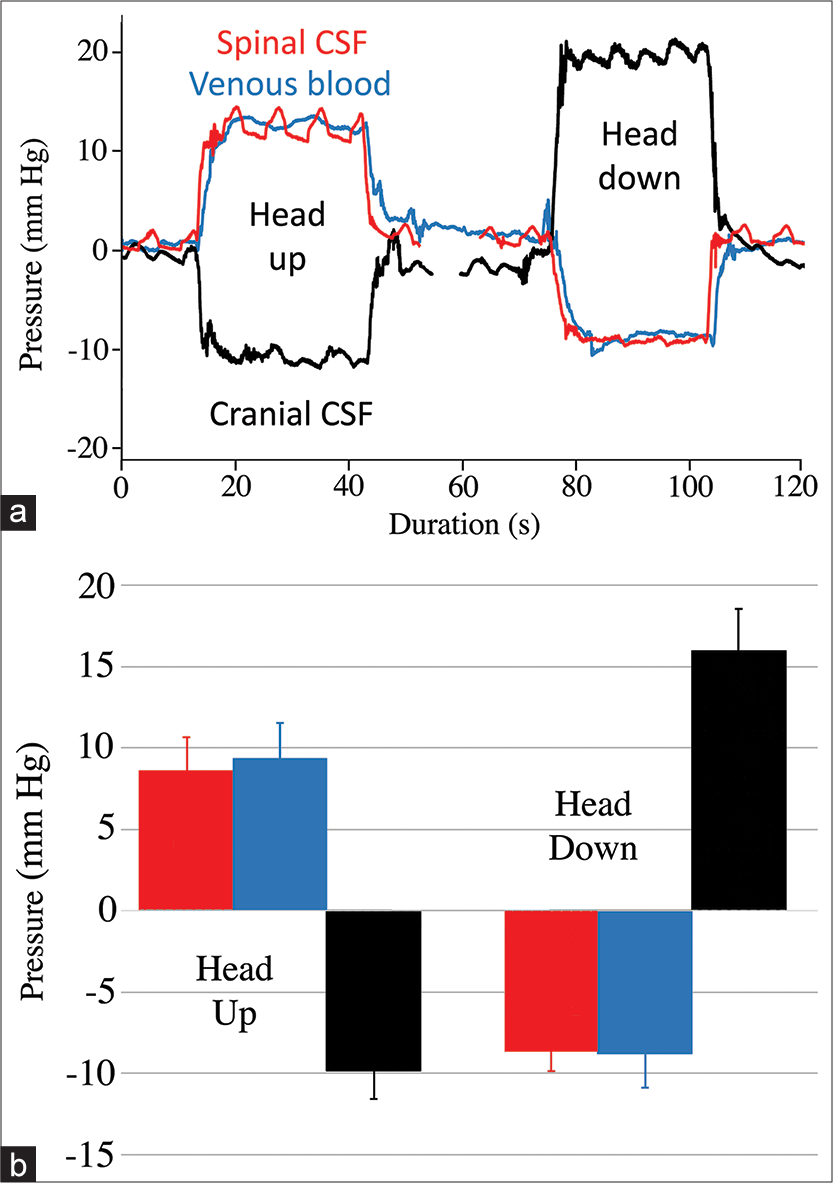
Figure 4:
Results from the orthostatic trials. (a) Composite raw data traces recorded from the same 158 cm Alligator mississippiensis. The spinal cerebrospinal fluid (CSF) pressure (red line) and venous blood pressure (blue line) exhibit a response similar in magnitude and direction; while the cranial CSF pressure (black line) responds in the opposite direction. The cranial CSF pressure is asymmetric showing a greater response to head-down rotations than to head-up rotations. (b) Graph of the orthostatic results; the height of the bars is the pooled mean for all of the trials, while the error bar represents one standard deviation. The spinal CSF pressure (red) and spinal venous pressure (blue) values were not significantly different; the cranial CSF pressures (black) were significantly different even after rectification.
If the pressure heads [
Push/pull experiments
When the cart was accelerated forward (pushed) the CSF pressure increased in the spinal region while decreasing in the cranial region; stopping the cart resulted in a negative spike in spinal CSF pressure and a positive spike in cranial CSF pressure [
Figure 6:
Raw data traces from two consecutive push-pull trials. Note that whether the alligator is pulled (a) or pushed (b) the cranial cerebrospinal fluid (CSF) pressure (black line) and spinal CSF pressure (red line) respond in opposite directions. The amplitude of the cranial CSF pressure changes (both peak and total) was significantly greater than those from the spinal CSF pressure.
Figure 7:
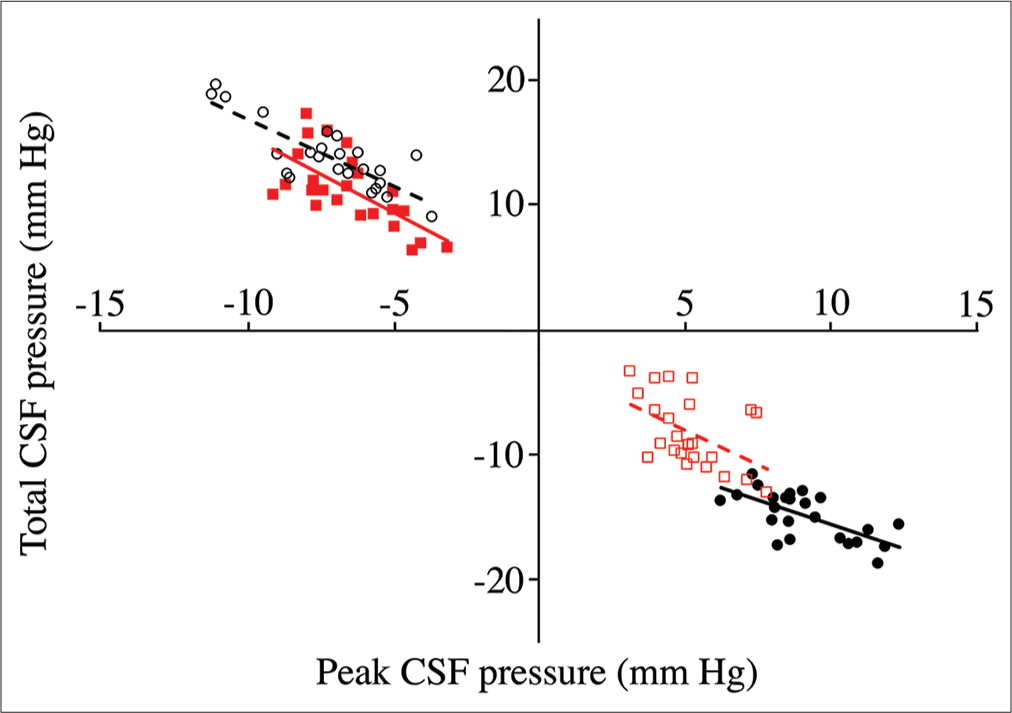
Relation of peak cerebrospinal fluid (CSF) pressure (X-axis) to total CSF pressure (Y-axis) from pooled trials. Data groups are presented as follows: cranial pulls (solid black circles, solid line); cranial push (open black circles, dashed line); spinal pulls (solid red squares, solid line); and spinal push (open red squares, dotted line). As expected, the direction of change in CSF pressure is always opposite in the cranial and spinal regions, opposite in the different directions of displacement, and opposite between peak and total. The cranial CSF pressures recorded during pulls have a different relationship between peak and total pressure than the other three data sets.
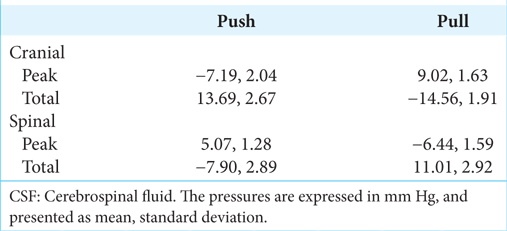
MANOVA of the rectified peak pressures [
The peak instantaneous velocity determined from the video analysis of the push/pull trials was multiplied by the mass of each animal to get momentum (in Newton-seconds, Ns). The pull trials had a pooled mean momentum of 22 Ns (standard deviation, s.d. = 6.2) while the push trials had a pooled mean of 18.97 Ns (s.d. = 6.4). A t-test revealed that the momentums present during the two trials were not significantly different (t = 1.68, P = 0.099).
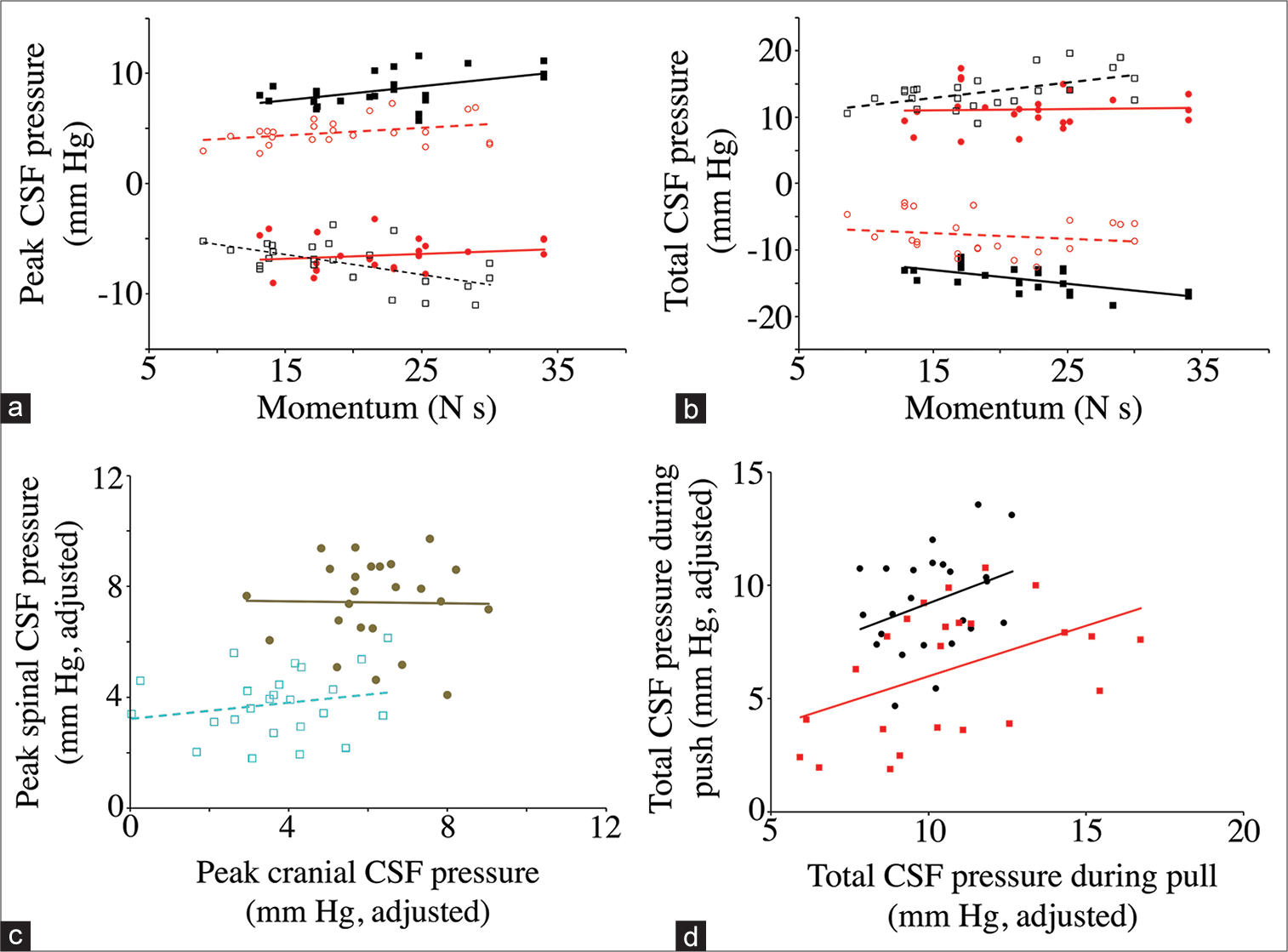
When the peak [
Figure 8:
Dynamic asymmetry in the push-pull data. The relationship between momentum and peak (a) and total (b) cerebrospinal fluid (CSF) pressure. Data groups are presented as follows: cranial pulls (solid black squares, solid line); cranial push (open black squares, dotted line); spinal pulls (solid red circles, solid line); and spinal push (open red circle, dashed line). As expected, the direction of change in CSF pressure is always opposite in the cranial and spinal regions, and opposite in the different directions of displacement. Note that the cranial values are larger than the spinal values, and that the regression lines for the cranial data have larger slopes than do those for the spinal data. (c) Relation of peak CSF pressure measured in the skull (X-axis) to peak CSF pressure measured along the spinal cord (Y-axis). These data have been adjusted to eliminate the variation attributable to momentum. Rearward displacement (pull) is indicated by solid brown markers, forward displacement (push) is indicated by open light blue markers. Note that pulling the animal produced greater CSF pressures, particularly in the spinal region. (d) Relation of total CSF pressure measured during rearward displacement (X-axis) to total CSF pressure measured during forward displacement (Y-axis). These data have been adjusted to eliminate the variation attributable to momentum. Cranial data are black circles, spinal data are red squares. Note that the cranial CSF pressures are generally greater than those measured from the spinal cord, though both data sets have a similar regression relationship.
Secondary pulses
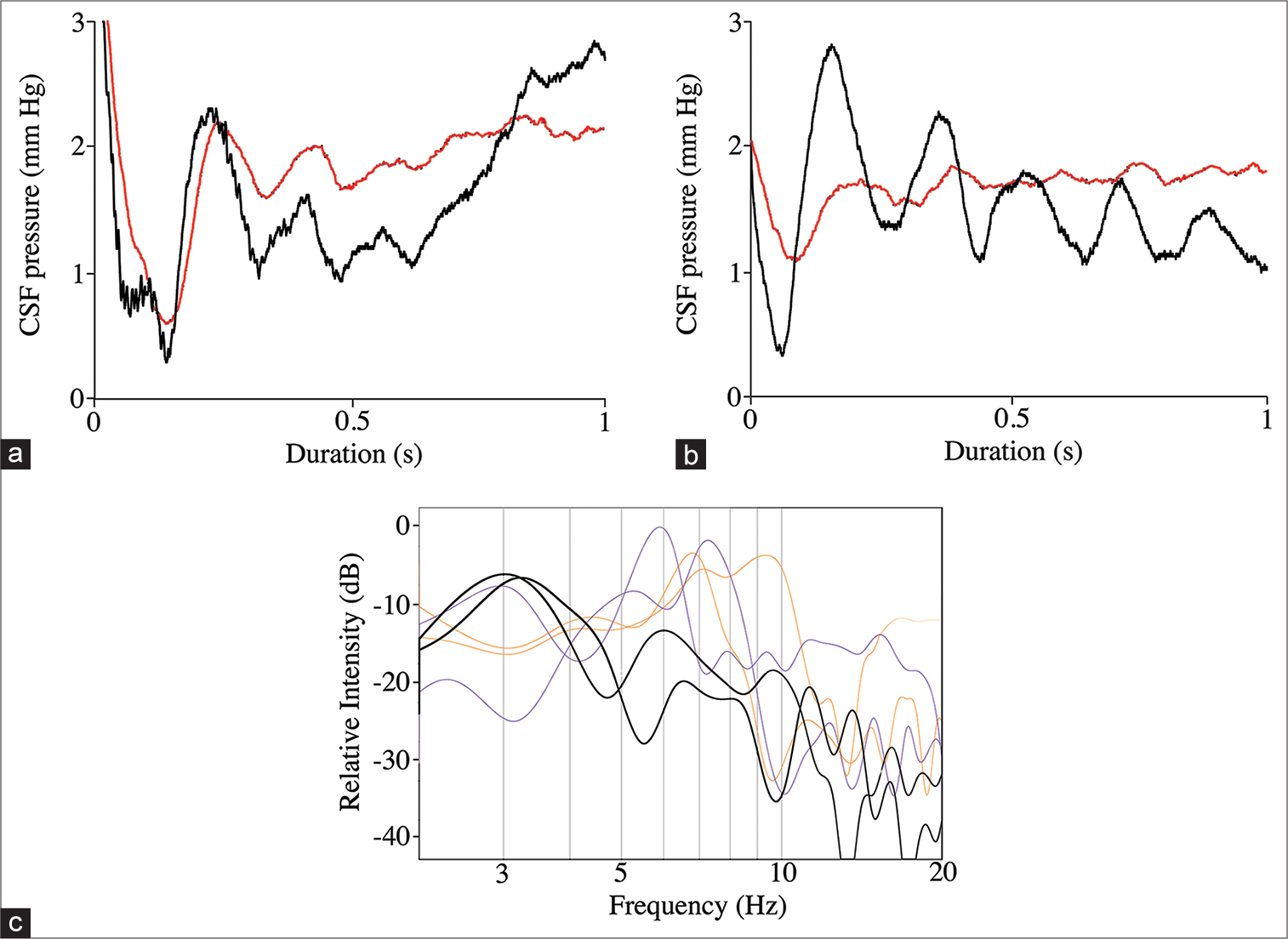
Secondary pulses in the CSF were observed following all of the experimental manipulations [
Figure 9:
Secondary pulses associated with the push-pull trials. (a) Representative data trace showing the cranial (black line) and spinal (red line) pressure pulses recorded immediately after the total pressure curve. The curves were rectified to facilitate viewing, but are otherwise raw data. Note the close temporal and amplitude patterns of the spinal and cranial secondary cerebrospinal fluid (CSF) pulses. (b) Representative data trace showing the cranial (black line) and spinal (red line) pressure pulses recorded immediately after the total pressure curve. The curves were rectified to facilitate viewing, but are otherwise raw data. These data traces were recorded after the surgical myodural release. Note that after release, the spinal CSF secondary pulses have a significantly lower amplitude and a lower fundamental frequency. (c) Power spectral analysis of the secondary pulses. The spinal and cranial CSF secondary pulses recorded before myodural release (thin lines) are not significantly different and have mean fundamental frequencies near 7 Hz; the spinal CSF secondary pulses (thick lines) recorded after myodural release has significantly lower fundamental frequencies (near 3 Hz).
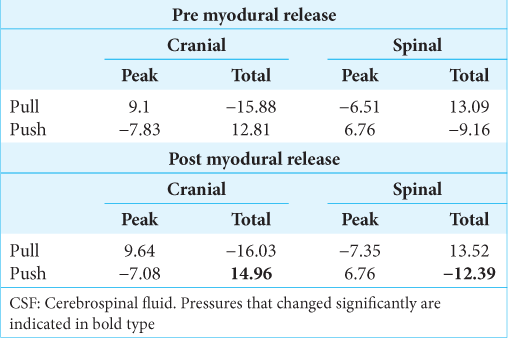
Myodural release
The myodural release had no significant influence on the peak pressures, nor any of the pressures produced by pulling the alligator backward [
DISCUSSION
Due to the relatively closed nature of the CSF and venous systems, and the integrity of the vertebrate heart, orthostatic gradients in vertebrates resolve around the hydrostatic indifference point of the body, not the axis of rotation.[
If a compensatory system is functioning during the head-down rotations, it is not as effective. While the spinal pressure and spinal venous blood pressure changes are symmetrical, the increase in cranial CSF pressure observed during head-down rotation is significantly greater than the decrease in pressure with head-up rotation [
The push/pull experiments were designed to create an impulse acting on the CSF. The preparation was first accelerated in one direction; the inertia of the CSF caused it to accumulate opposite the direction of the acceleration (causing differential “peak” readings at the two CSF pressure sensors). When the preparation was then rapidly stopped, the resulting impulse caused the CSF to flow opposite the initial movement (causing a reversal in both CSF pressure sensors, the “total” values, which were always greater than the initial peak pressures). Inertial shifts in the CNS are a well-known source of neural trauma.[
The push/pull trials consistently yielded asymmetric results; the pressure change in the cranial compartment was significantly greater than the pressure change in the spinal compartment [
Both the orthostatic gradient and push/pull experiments yielded asymmetric results in which cranial CSF pressures and/or cranial CSF pressure changes were significantly greater than spinal CSF pressures. We hypothesize that the dynamic asymmetry results from differential compliance. Previous experimental studies in humans have used the introduction of extra fluids (infusion studies) or surgical openings in the system to manipulate the compliance of the dura.[
The relationship between spinal and cranial dural compliance in Alligator is likely more variable than it is in humans, due to the presence of the large spinal venous sinus in Alligator [
There are (at least) three important limitations to this study. This study examined only CSF pressure, not CSF flow. Herein, it is assumed that the cranial and spinal CSF was equally influenced by the orthostatic gradients and push/pull trials. It takes some 3500 mm Hg of pressure to induce cavitation in CSF;[
Regardless of which experimental means was used to produce the pulses of CSF pressure, the pulses were consistently followed by “secondary pulses.” These secondary pulses were of much lower amplitude [
The myodural bridge of Alligator is well-developed, and contraction of the myodural bridge changes the CSF pressure.[
CONCLUSION
External forces, applied as gravitational gradients or linear accelerations, resulted in differential pressure changes in the cranial and spinal (CSF). Herein the induced differential changes in CSF pressure are interpreted as reflecting differential compliance within the cranial and spinal compartments. The results suggest that in Alligator, the spinal compartment has higher dural compliance than the cranial compartment (which is opposite the situation found in humans); the spinal dural compliance of Alligator is likely determined by the presence of a large spinal venous sinus. Differential blood pressure within the spinal sinus, like differential activation of the myodural bridge, has the potential to modulate the compliance of the spinal compartment and influence CSF exchange between the brain and spinal cord.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgements
The authors wish to thank the Louisiana Department of Wildlife and Fisheries who kindly provided the alligators used in this study, and P. Kondrashov for his continued support of this research program. Figure 1a was skillfully rendered by Jamie Carroll.
References
1. Alperin N, Hushek SG, Lee SH, Sivaramakrishnan A, Lichtor T. MRI study of cerebral blood flow and CSF flow dynamics in an upright posture: The effect of posture on the intracranial compliance and pressure. Acta Neurochir Suppl. 2005. 95: 177-81
2. Anile C, Bonis PD, Ficola A, Santini P, Mangiola A. An experimental study on artificially induced CSF pulse waveform morphological modifications. Neurol Res. 2011. 33: 1072-82
3. Ariens Kappers C. The meninges in lower vertebrates compared with those in mammals. Arch Neurol Psychiatry. 1926. 15: 281-96
4. Bruner HL. On the cephalic veins and sinuses of reptiles: With description of a mechanism for raising the venous blood-pressure in the head. Am J Anat. 1907. 7: 1-117
5. Bustamante MC, Cronin DS. Cavitation threshold evaluation of porcine cerebrospinal fluid using a Polymeric Split Hopkinson Pressure Bar-Confinement chamber apparatus. J Mech Behav Biomed Mater. 2019. 100: 103400
6. Charlton JD, Johnson RN, Pederson NE, Mann JD. Assessment of cerebrospinal fluid compliance and outflow resistance: Analysis of steady-state response to sinusoidal input. Ann Biomed Eng. 1983. 11: 551-61
7. Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004. 75: 813-821
8. de Oliveira E, Rhoton AL, Peace D. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1985. 24: 293-352
9. Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gärtner J, Frahm J. Identification of the upward movement of human CSF in vivo and its relation to the brain venous system. J Neurosci. 2017. 37: 2395-402
10. Fyneface-Ogan S, editors. Anatomy and clinical importance of the epidural space. Epidural Analgesia-Current Views and Approaches. London, UK: Intechopen; 2012. 12: 1-12
11. Greer S, Cramberg MJ, Young BA. Morphometrics of the spinal cord and surrounding structures in Alligator mississippiensis. Biology (Basel). 2022. 11: 514
12. Grondel B, Cramberg M, Greer S, Young BA. The morphology of the suboccipital region in snakes, and the anatomical and functional diversity of the Myodural bridge. J Morphol. 2022. 283: 123-33
13. Hack GD, Koritzer RT, Robinson WL, Hallgren RC, Greenman PE. Anatomic relation between the rectus capitis posterior minor muscle and the dura mater. Spine (Phila Pa 1976). 1995. 20: 2484-6
14. Hinghofer-Szalkay H. Gravity, the hydrostatic indifference concept and the cardiovascular system. Eur J Appl Physiol. 2011. 111: 163-74
15. Holm S, Eide PK. The frequency domain versus time domain methods for processing of intracranial pressure (ICP) signals. Med Eng Phys. 2008. 30: 164-70
16. Holmlund P, Johansson E, Qvarlander S, Wåhlin A, Ambarki K, Koskinen LD. Human jugular vein collapse in the upright posture: Implications for postural intracranial pressure regulation. Fluids Barriers CNS. 2017. 14: 17
17. Ito K, Yamada M, Horiuchi T, Hongo K. Microanatomy of the dura mater at the craniovertebral junction and spinal region for safe and effective surgical treatment. J Neurosurg Spine. 2020. 33: 165-71
18. Kasprowicz M, Lalou DA, Czosnyka M, Garnett M, Czosnyka Z. Intracranial pressure, its components and cerebrospinal fluid pressure-volume compensation. Acta Neurol Scand. 2016. 134: 168-80
19. Klarica M, Kuzman T, Jurjević I, Radoš M, Tvrdeić A, Orešković D. The effect of body position on intraocular and intracranial pressure in rabbits. Acta Neurochir Suppl. 2016. 122: 279-82
20. Knoche L, Young BA, Kondrashova T. The influence of gravitational gradients on the American alligator (Alligator mississippiensis). Anat Physiol Curr Res. 2019. 9: 318
21. Kondrashova T, Blanchard J, Knoche L, Potter J, Young BA. Intracranial pressure in the American alligator (Alligator mississippiensis): Reptilian meninges and orthostatic gradients. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. 206: 45-54
22. Kramer LA, Hasan KM, Sargsyan AE, Marshall-Goebel K, Rittweger J, Donoviel D. Quantitative MRI volumetry, diffusivity, cerebrovascular flow, and cranial hydrodynamics during head-down tilt and hypercapnia: The SPACECOT study. J Appl Physiol (1985). 2017. 122: 1155-66
23. Lai HX, Gong J, Hack GD, Song TW, Liu B, Yu SB. Development, maturation, and growth of the myodural bridge within the posterior atlanto-axial interspace in the rat. J Morphol. 2022. 283: 993-1002
24. Linninger AA, Tangen K, Hsu CY, Frim D. Cerebrospinal fluid mechanics and its coupling to cerebrovascular dynamics. Ann Rev Fluid Mech. 2016. 48: 219-57
25. Magnaes B. Body position and cerebrospinal fluid pressure. Part 2: Clinical studies on orthostatic pressure and the hydrostatic indifferent point. J Neurosurg. 1976. 44: 698-705
26. Marmarou A, Shulman K, LaMorgese J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg. 1975. 43: 523-34
27. Matsumae M, Kuroda K, Yatsushiro S, Hirayama A, Hayashi N, Takizawa K. Changing the currently held concept of cerebrospinal fluid dynamics based on shared findings of cerebrospinal fluid motion in the cranial cavity using various types of magnetic resonance imaging techniques. Neurol Med Chir (Tokyo). 2019. 59: 133-46
28. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011. 13: 287-300
29. Namjoshi DR, Good C, Cheng WH, Panenka W, Richards D, Cripton PA. Towards clinical management of traumatic brain injury: A review of models and mechanisms from a biomechanical perspective. Dis Model Mech. 2013. 6: 1325-38
30. Nitta M, Kasuga Y, Hasegawa Y, Nagai H, editors. Conduction time of the pulse through the brain with increased intracranial pressure. Intracranial Pressure. Berlin: Springer; 1983. 5: 211-5
31. Palomeque-Del-Cerro L, Arráez-Aybar LA, Rodríguez-Blanco C, Guzmán-García R, Menendez-Aparicio M, Oliva-Pascual-Vaca Á. A systematic review of the soft-tissue connections between neck muscles and dura mater: The myodural bridge. Spine (Phila Pa 1976). 2017. 42: 49-54
32. Pearcy Q, Jeejo M, Scholze M, Tomlinson J, Dressler J, Zhang M. Biomechanics of vascular areas of the human cranial dura mater. J Mech Behav Biomed Mater. 2022. 125: 104866
33. Qvarlander S, Malm J, Eklund A. CSF dynamic analysis of a predictive pulsatility-based infusion test for normal pressure hydrocephalus. Med Biol Eng Comput. 2014. 52: 75-85
34. Qvarlander S, Sundström N, Malm J, Eklund A. Postural effects on intracranial pressure: Modeling and clinical evaluation. J Appl Physiol (1985). 2013. 115: 1474-80
35. Song X, Gong J, Yu SB, Yang H, Song Y, Zhang XH. The relationship between compensatory hyperplasia of the myodural bridge complex and reduced compliance of the various structures within the cranio-cervical junction. Anat Rec (Hoboken). 2023. 306: 401-8
36. Takizawa H, Gabra-Sanders T, Miller JD. Changes in the cerebrospinal fluid pulse wave spectrum associated with raised intracranial pressure. Neurosurgery. 1987. 20: 355-61
37. Wagshul ME, Eide PK, Madsen JR. The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS. 2011. 8: 5
38. Xu Q, Yu SB, Zheng N, Yuan XY, Chi YY, Liu C. Head movement, an important contributor to human cerebrospinal fluid circulation. Sci Rep. 2016. 6: 31787
39. Young BA, Adams J, Beary JM, Mardal KA, Schneider R, Kondrashova T. The myodural bridge of the American alligator (Alligator mississippiensis) alters CSF flow. J Exp Biol. 2020. 223: jeb230896
40. Young BA, Cramberg MJ. Treadmill locomotion in the American alligator (Alligator mississippiensis) produces dynamic changes in intracranial cerebrospinal fluid pressure. Sci Rep. 2022. 12: 11826
41. Young BA, Cramberg M. The influence of movement on the cerebrospinal fluid pressure of the American alligator (Alligator mississippiensis). Biology (Basel). 2022. 11: 1702
42. Zippel KC, Lillywhite HB, Mladinich CR. Anatomy of the crocodilian spinal vein. J Morphol. 2003. 258: 327-35