- Department of Neurosurgery, Yokohama Sakae Kyosai Hospital, Yokohama, Kanagawa, Japan
- Department of Neurosurgery, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa, Japan
- Department of Neurosurgery, Shima Neurological Orthopedic Hospital, Kawasaki, Japan
Correspondence Address:
Keita Fujii, Department of Neurosurgery, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa, Japan.
DOI:10.25259/SNI_768_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Keita Fujii1,2, Kentaro Mori1, Akira Tamase1, Hiroshi Shima3, Motohiro Nomura1, Tetsuya Yamamoto2. Dynamic changes of abnormal muscle response during decompression procedures in double compression-type hemifacial spasm. 22-Nov-2024;15:430
How to cite this URL: Keita Fujii1,2, Kentaro Mori1, Akira Tamase1, Hiroshi Shima3, Motohiro Nomura1, Tetsuya Yamamoto2. Dynamic changes of abnormal muscle response during decompression procedures in double compression-type hemifacial spasm. 22-Nov-2024;15:430. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13243
Abstract
Background: Hemifacial spasm (HFS) is a neurovascular movement caused by vascular compression of the facial nerve in its root exit zone (REZ). Cases of HFS caused by double compression (DC) in both REZ and the cisternal portion (CP) have been sporadically reported. The nature of DC-type HFS is still not fully understood. Compression in CP is often overlooked, resulting in reoperation in DC-type HFS cases.
Case Description: A 48-year-old man with a 3-year history of left HFS was admitted to our department. Magnetic resonance imaging revealed that the vertebral artery (VA) passed around REZ of the facial nerve, and the anterior inferior cerebellar artery (AICA) was in contact with the facial nerve in CP. Microvascular decompression was performed while monitoring any abnormal muscle response (AMR). Although VA was dissected and detached from REZ, AMR showed only a transient decrease and the amplitude of the AMR wave soon recovered and subsequently increased. No other vessels compressing REZ beneath VA were found. AICA attached to the facial nerve in CP and was compressed upward by VA. When AICA was moved from the facial nerve in CP after the transposition of VA, AMR was immediately resolved. After surgery, the patient was completely free from HFS.
Conclusion: In DC-type HFS, precise preoperative diagnosis and intraoperative identification of the culprit vessel are difficult. In DC-type HFS, decompression of one side of a vessel may exacerbate the compression of the other side. In such a case, AMR helps us become aware of compressions in CP that we may preoperatively overlook. AMR is useful for identifying the exact culprit vessels and recognizing any compression changes caused by intraoperative manipulations.
Keywords: Abnormal muscle response, Anterior inferior cerebellar artery, Cisternal portion, Hemifacial spasm, Microvascular decompression, Vertebral artery
INTRODUCTION
Hemifacial spasm (HFS) is usually caused by compression of the facial nerve by blood vessels in the root exit zone (REZ). Microvascular decompression (MVD) is widely known as a treatment for HFS. Good outcomes and low complication rates have been reported.[
Real-time abnormal muscle response (AMR) monitoring has been used to assess the adequacy of facial nerve decompression. AMR can predict postoperative symptom resolution.[
Recently, we encountered a case of DC-type HFS in which AICA in CP was considered to be the direct culprit vessel. In our case, the AMR amplitude did not completely disappear but rather exacerbated after the release of nerve compression by VA in REZ alone. Translocation of the thick VA in REZ might exacerbate tandem compression of AICA by the distal VA in CP. This anatomical change of vessels might result in exacerbation of compression of the facial nerve by AICA in CP. We report a case of DC-type HFS caused by VA and AICA, showing dynamic changes in the AMR wave in which AMR was effectively utilized.
CASE REPORT
A 48-year-old man with a 3-year history of the left HFS was admitted to our department. His facial spasms gradually increased in frequency and became persistent almost all day long. The spasm was particularly aggravated under stress, and he sometimes could not open his eyes. He had no previous medical history. Preoperative neurological examinations revealed no abnormal findings other than HFS. On magnetic resonance imaging, bilateral VAs were thick, and thicker right VA severely deviated to the left. The tortuous left VA passed around and compressed the REZ of the facial nerve. AICA originated from the basilar artery and ran along the left VA close to the proximal portion of the facial nerve. AICA was in contact with the facial nerve in CP [
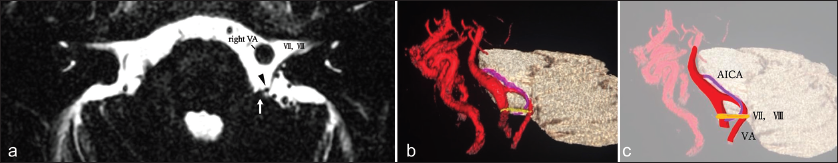
Figure 1:
(a) Constructive interference in steady state imaging of magnetic resonance imaging (MRI) revealing vascular compression at the root exit zone by the left vertebral artery (VA) (arrow) and at the cisternal portion by anterior inferior cerebellar artery (AICA) (arrowhead). (b) MRI facilitates 3D confirmation of the relationships between vessels and nerves. (c) Schematic drawing of 3D MRI. VII: Facial nerve, VIII: Vestibulocochlear nerve.
Lateral suboccipital craniotomy was conducted. The left VA was thick and hard. The left VA ran beneath the facial nerve. VA was on REZ of the facial nerve and also compressed the trigeminal nerve. AICA runs along the VA and is in contact with the facial nerve [
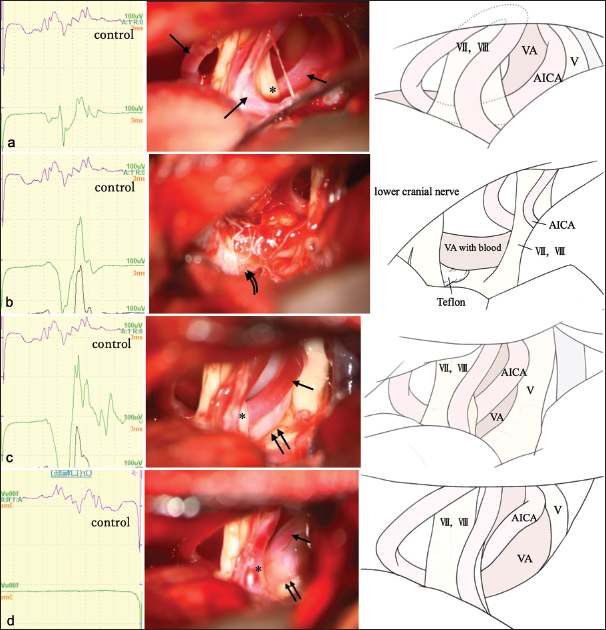
Figure 2:
Abnormal muscle response (AMR) waves (left panels), intraoperative photographs (mid), and schematic illustrations (right). (a) Intraoperative findings before microscopic manipulation. The anterior inferior cerebellar artery (AICA) (arrow) on the vertebral artery (VA) is in contact with the facial nerve (asterisk). (b) AMR waves (left panels), intraoperative photographs (mid), and schematic illustrations (right). Teflon felt (curved double arrows) is inserted between the brain stem and VA. AMR is amplified after decompression of the root exit zone. (c) AMR waves (left panels), intraoperative photographs (mid), and schematic illustrations (right). Compression of the facial nerve by AICA (arrow) is exacerbated after further decompression of both sides of the facial nerve (asterisk). AMR is further amplified. (d) AMR waves (left panels), intraoperative photographs (mid), and schematic illustrations (right). Compression of the facial nerve by AICA (arrow) is completely resolved by transposition using fibrin glue (asterisk). AMR remains absent after surgery. double arrows: VA, V: Trigeminal nerve, VII: Facial nerve, VIII:Vestibulocochlear nerve.
DISCUSSION
HFSs are basically due to the compression of nerves caused by beating blood vessels in REZ.[
In DC-type HFS, the vessel most frequently compressing CP is AICA (97%). AICA being compressed by VA in CP was considered to be the cause of HFS in our case. VA was found in REZ and simultaneously contributed to the compression of AICA in CP. This means that the thick VA contacted AICA in CP and contributed to the compression of the facial nerve. A translocated VA in REZ might exacerbate compression of AICA in CP after the decompression procedure in REZ [
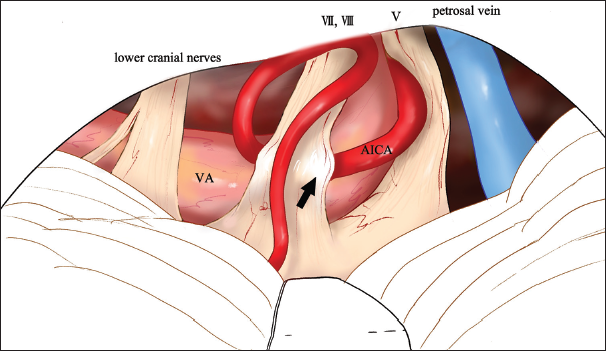
Figure 3:
Overview illustration representing compression of the facial nerve by vertebral artery (VA) and anterior inferior cerebellar artery (AICA). Transposition of VA and decompression of the root exit zone exacerbated facial nerve compression at the cisternal portion through the AICA (arrow). V: trigeminal nerve, VII: Facial nerve, VIII: Vestibulocochlear nerve.
AMR has been used in MVD for HFS to assess whether adequate decompression has been achieved. Intraoperative AMR monitoring exhibits high specificity for predicting a spasm-free status after MVD. AMR is also useful in cases of compression in CP. Zheng et al. reported that if AMR had not been used, at least 17 of 20 cases of multi-compression would have been missed.[
CONCLUSION
In DC-type HFS, decompression of the vessel on one side may exacerbate compression on the other side as a lever, especially in cases with thick and atherosclerotic vessels. AMR is useful to indicate the existence of a culprit vessel in CP that may have been preoperatively overlooked. It is also helpful to recognize anatomical changes regarding the relationship between nerves and vessels.
Ethical approval
All procedures performed in this study involving human participants were conducted in accordance with ethical standards of the Institutional and/or National Research Committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We would like to express our sincere thanks to Issei Fukui (Department of Neurosurgery, Yokohama Sakae Kyosai Hospital), Ryo Yamada, Mari Kojima, and Tatsu Nakano (Department of Neurology, Yokohama Sakae Kyosai Hospital), and Kazushige Sakuda (Department of Neurosurgery, Sakuda Neurosurgery Clinic, Fujisawa, Japan) for their valuable contributions to this study. Their support in data collection, analysis, and manuscript preparation was essential to the successful completion of this research.
References
1. Campos-Benitez M, Kaufmann AM. Neurovascular compression findings in hemifacial spasm. J Neurosurg. 2008. 109: 416-20
2. Chang WS, Kim HY, Chung SS, Chang JW. Microneurovascular decompression in patients with hemifacial spasm caused by vascular compression of facial nerve at cisternal portion. Acta Neurochir (Wien). 2010. 152: 2105-1
3. Fukuda M, Kameyama S, Honda Y, Tanaka R. Hemifacial spasm resulting from facial nerve compression near the internal acoustic meatus-case report. Neurol Med Chir (Tokyo). 1997. 37: 771-4
4. Hirono S, Yamakami I, Sato M, Kado K, Fukuda K, Nakamura T. Continuous intraoperative monitoring of abnormal muscle response in microvascular decompression for hemifacial spasm; a real-time navigator for complete relief. Neurosurg Rev. 2014. 37: 311-9 discussion 319-20
5. Jannetta PJ. Microsurgery of cranial nerve cross-compression. Clin Neurosurg. 1979. 26: 607-15
6. Kim JP, Park BJ, Choi SK, Rhee BA, Lim YJ. Microvascular decompression for hemifacial spasm associated with vertebrobasilar artery. J Korean Neurosurg Soc. 2008. 44: 131-5
7. Li S, Hong W, Tang Y, Ying T, Zhang W, Li X. Re-operation for persistent hemifacial spasm after microvascular decompression with the aid of intraoperative monitoring of abnormal muscle response. Acta Neurochir (Wien). 2010. 152: 2113-8
8. Liu MX, Zhong J, Xia L, Dou NN, Sun H, Li B. The significance of abnormal muscle response monitoring during microvascular decompression for hemifacial spasm. Acta Neurochir Suppl. 2017. 124: 297-301
9. Masuoka J, Matsushima T, Nakahara Y, Inoue K, Yoshioka F, Kawashima M. Outcome of microvascular decompression for hemifacial spasm associated with the vertebral artery. Neurosurg Rev. 2017. 40: 267-73
10. Mikami T, Minamida Y, Akiyama Y, Wanibuchi M, Sugino T, Houkin K. Microvascular decompression for hemifacial spasm associated with the vertebral artery. Neurosurg Rev. 2013. 36: 303-8 discussion 308-9
11. Nagahiro S, Takada A, Matsukado Y, Ushio Y. Microvascular decompression for hemifacial spasm. Patterns of vascular compression in unsuccessfully operated patients. J Neurosurg. 1991. 75: 388-92
12. Ryu H, Yamamoto S, Sugiyama K, Uemura K, Miyamoto T. Hemifacial spasm caused by vascular compression of the distal portion of the facial nerve. Report of seven cases. J Neurosurg. 1998. 88: 605-9
13. Samii M, Gunther T, Iaconetta G, Muehling M, Vorkapic P, Samii A. Microvascular decompression to treat hemifacial spasm: Long-term results for a consecutive series of 143 patients. Neurosurgery. 2002. 50: 712-8 discussion 718-9
14. Son BC, Ko HC, Choi JG. Hemifacial spasm caused by vascular compression in the cisternal portion of the facial nerve: Report of two cases with review of the literature. Case Rep Neurol Med. 2019. 2019: 8526157
15. Yang DB, Wang ZM. Microvascular decompression for hemifacial spasm associated with bilateral vertebral artery compression. Acta Neurol Belg. 2017. 117: 713-17
16. Zheng X, Feng B, Zhang W, Ying T, Li S. Hemifacial spasm caused by cross type vascular compression. Neurol Res. 2011. 33: 965-9
17. Zhong J, Li ST, Zhu J, Guan HX. Is entire nerve root decompression necessary for hemifacial spasm?. Int J Surg. 2011. 9: 254-7
18. Zhong J, Zhu J, Li ST, Li XY, Wang XH, Yang M. An analysis of failed microvascular decompression in patients with hemifacial spasm: Focused on the early reoperative findings. Acta Neurochir (Wien). 2010. 152: 2119-23