- Department of Neurosurgery, University of California, Irvine, Orange, United States
Correspondence Address:
Nolan Kyle Winslow, Department of Neurosurgery, University of California, Irvine, Orange, United States.
DOI:10.25259/SNI_277_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nolan Kyle Winslow, Alexander Scott Himstead, Sumeet Vadera. Early case series with placement of NeuroOne Evo stereoelectroencephalography depth electrodes and review of other Food and Drug Administration-approved products. 06-Dec-2024;15:454
How to cite this URL: Nolan Kyle Winslow, Alexander Scott Himstead, Sumeet Vadera. Early case series with placement of NeuroOne Evo stereoelectroencephalography depth electrodes and review of other Food and Drug Administration-approved products. 06-Dec-2024;15:454. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13273
Abstract
Background: Stereoelectroencephalography (SEEG) is a common diagnostic surgical procedure for patients with medically refractory epilepsy. We aimed to describe our initial experience with the recently released NeuroOne Evo SEEG electrode product (Zimmer Biomet, Warsaw, IN) and review technical specifications for other currently approved depth SEEG electrodes.
Methods: We performed a record review on the first five patients implanted with NeuroOne Evo SEEG electrode product using the robotic stereotactic assistance robot platform and described our surgical technique in detail. We recorded technical specifications of all currently Food and Drug Administration-approved SEEG electrodes for comparison.
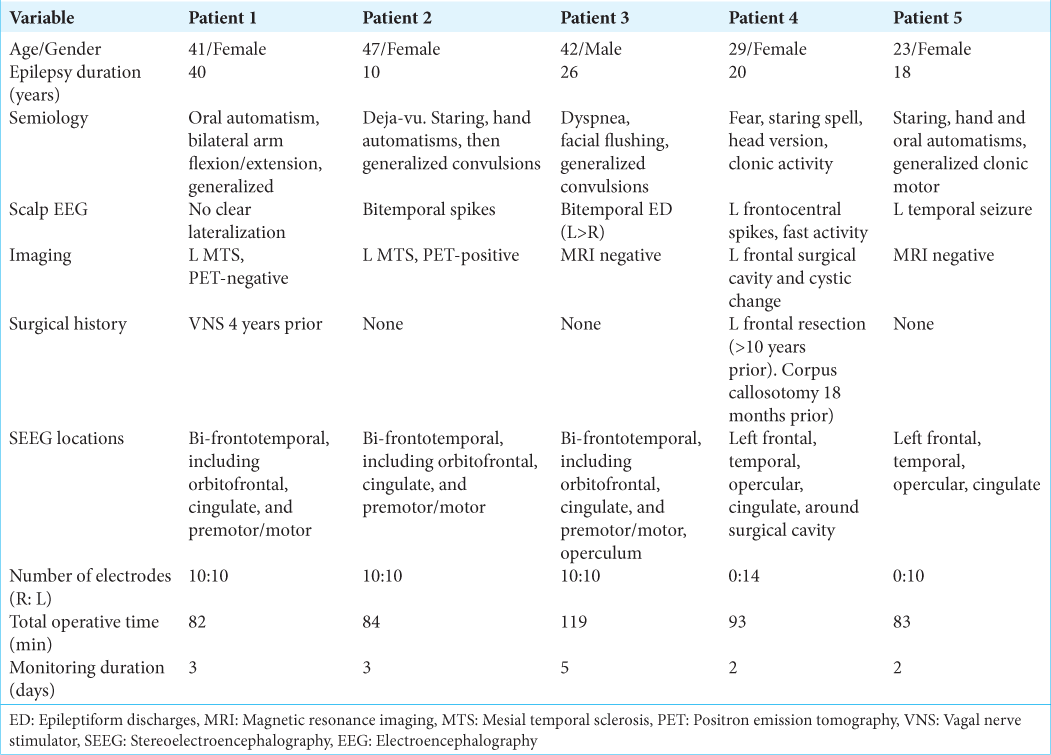
Results: Our initial 5 surgical patients were reviewed. The average total time of operation was 92 min, with an average of 16.8 electrodes. The estimated time per electrode insertion was
Conclusion: NeuroOne SEEG electrodes can be implanted with efficiency and provide a valuable additional tool for the epilepsy surgeon. A tapered drill bit prevents the bolt from being placed beyond the inner cortex and may reduce the risk of brain contusion or inadvertent advancement of anchor bolts, and the electrode internal stylet also affords the potential to reduce the number of trajectory passes.
MeSH Terms: Epilepsy, EEG, Drug-resistant Epilepsy, Intracranial EEG
Keywords: AdTech, DiXi, NeuroOne, Positron emission tomography (PMT), Stereoelectroencephalography
INTRODUCTION
Stereoelectroencephalography (SEEG) is a widely utilized technique in invasive monitoring for medically refractory epilepsy when less invasive techniques are unable to distinguish potential epileptogenic areas effectively. In this procedure, multiple electrodes are surgically implanted into the brain using one of several targeting methods. Stereotactic head frames and surgical robots are common tools used for insertion. Robotic insertion has been shown to lower average operative time and perhaps improve accuracy.[
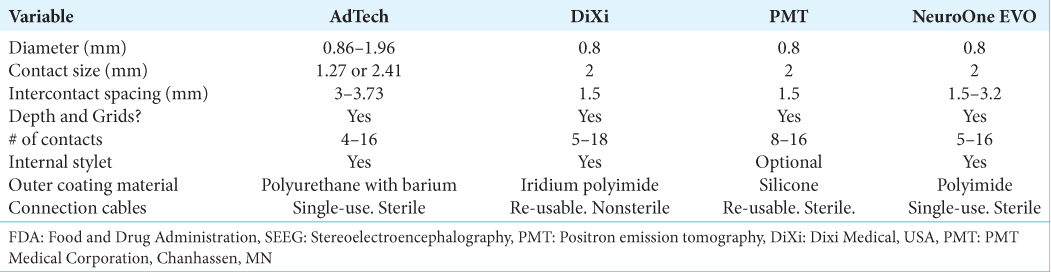
Currently, Food and Drug Administration-approved SEEG electrode products include those from AdTech (Oak Creek, WI), PMT (Chanhassen, MN), DiXI (Chaudefontaine, France), and NeuroOne (Zimmer Biomet, Warsaw, IN, USA) companies. NeuroOne is the most recently approved of these, and little has been published about this electrode system to date.[
SEEG is becoming a more and more prevalent method of intracranial monitoring when advanced diagnostics are required to localize a patient’s epilepsy. Due to the minimally invasive nature of SEEG, our center frequently employs this technique in refractory epilepsy that scalp electroencephalography (EEG) is unable to categorize confidently. The NeuroOne electrode design provides for reduced steps during implantation and makes the process of implantation more efficient. The authors report our initial experience and the technical specifications of the NeuroOne electrodes, with the goal of making the reader aware of new devices in epilepsy surgery, which could enhance safety and reduce steps involved in the implantation process.
MATERIALS AND METHODS
We performed a case series review of our first five consecutive patients with Zimmer NeuroOne EVO SEEG electrodes inserted at the University of California, Irvine Douglas, over approximately 6 months in 2023. The clinical, radiographic, and surgical history of each epilepsy patient was reviewed retrospectively through medical record review [
Surgical and medical device photographs were collected without any identifying features or patient identifiers, and, as is standard practice at our institution, each patient has consented before surgery to the possibility of publishing any photographs or videos obtained in connection with their clinical and surgical information in a de-identified fashion. This study was performed in line with the principles of the Declaration of Helsinki. Local Institutional Review Board Approval was granted before study initiation. This case series has been reported in line with the PROCESS guideline.
Surgical procedure
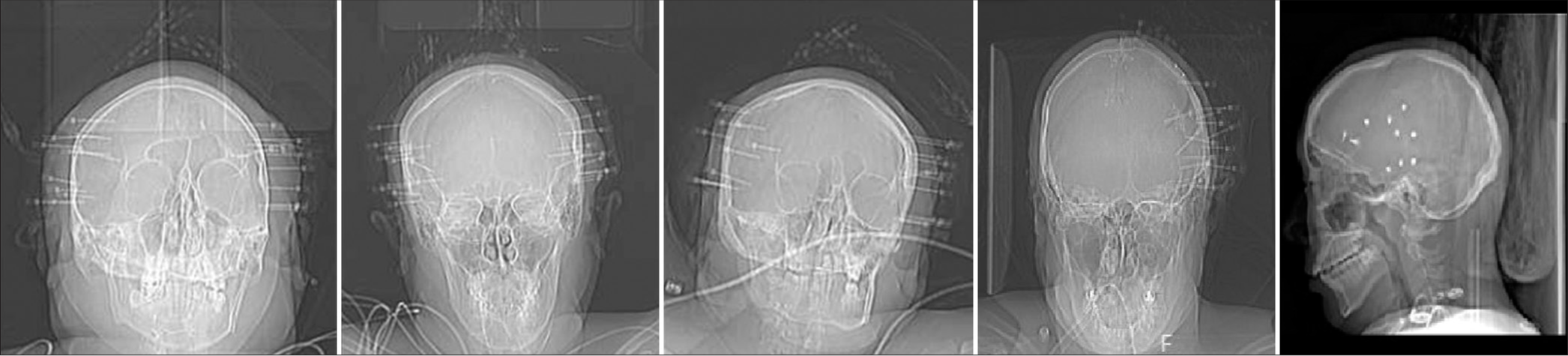
Procedures were performed under general endotracheal anesthesia. After preprocedural time-out and clipping of hair, the head was fixed into place with a Leksell stereotactic frame (Elekta Solutions, Sweden) or Mayfield skull clamp system (Integra Neurosciences, Plainsboro, NJ) and then attached to the robotic stereotactic assistance (ROSA) robot (Zimmer Biomet, Warsaw, IN, USA). Facial registration was performed using the built-in robotic software and laser capabilities, utilizing both preoperative computed tomography (CT) and double-contrast magnetic resonance imaging. This registration process takes between 20 and 40 min. Once the robot is calibrated, the patient is prepped and draped. Electrode trajectories were preplanned and loaded onto the robot. Variable-length NeuroOne electrodes were inserted with ROSA assistance through previously planned trajectories as described in the following text. Systolic arterial pressure was maintained below 130 mmHg for the duration of electrode insertion time. Antiplatelets and anticoagulants were held for multiple days before each procedure. Drilling into the skull was accomplished with a 2.1 mm tapered drill bit aimed through a robotic attachment piece along the trajectory for each electrode. The width of the bone at each entry point was measured on preoperative images, and the robotic attachment for drill guidance was used as a safety stop and positioned 3–5 mm beyond the anticipated bone width. With each increase in the depth of drilling, the surgeon would move the robot drill guide several millimeters along the trajectory and then advance the drill. In doing so, the surgeon was able to maintain manual feedback upon drilling through the inner table of the calvarium and perform one final 1–2 mm advancement of the drill guide (and subsequently, drill bit) with the intent to perforate through the dura mater with the drill tip. These drilling steps are illustrated in our associated surgical video [ An alternative to using the drill tip to perforate the dura is to use a separate probe with a tapered sharp endpoint combined with a cautery device to open the dura. With this method, the surgeon can also palpate the dura and use monopolar electrocautery periodically to transmit electricity through the palpation probe and create a small opening within the dura. Varying length bolts (20–35 mm) were placed into the predrilled hole based on the measured soft-tissue thickness at each location. Once into the bone, approximately 5 turns were performed to anchor each bolt. After dural perforation, each electrode was inserted. No preinserting stylet pass was used, as the NeuroOne Evo SEEG electrode has an incorporated internal stylet, which provides adequate rigidity for placement. Electrodes were planned and placed with an orthogonal trajectory whenever possible. Each electrode was anchored to a metal bolt fixated in the skull by tightening the electrode cap until finger tight. Each electrode had between 10 and 16 contacts. After insertion, electrodes were labeled, and sterile bandages were dressed along each exit site. Postoperative X-rays were obtained before leaving the operating room (OR) [
Video 1
RESULTS
Our initial five cases with NeuroOne EVO electrodes proceeded without any apparent technical issues. It is reasonable to expect an average time of <2 min per electrode insertion with this system. We did not have any intracranial hemorrhage on immediate postoperative CT scans. Monitoring yielded diagnostic information in all patients, and there were no apparent hardware complications. Surgical removal of the electrode and anchor bolt systems after monitoring proceeded without any complications, and incisions (closed with staples) were well healed at 2-week follow-up appointments for each patient.
DISCUSSION
There are several small alterations to surgical techniques utilized in our series that make electrode insertion more efficient. The use of a robot to aid in the efficiency and accuracy of electrode insertion has been documented previously.[
Other benefits of a built-in stylet model are the lack of need for a separate stylet, which adds cost to the procedure, and the avoidance of potential deformation or bending of a stylet with repeated use. A disposable stylet can be opened for each case should the surgeon desire to create an additional trajectory pass before inserting the SEEG electrode. As we accumulate experience with NeuroOne electrode insertion, we anticipate that high accuracy of placement will eliminate the need for utilizing this (or multiple) additional stylets and perhaps reduce cost.
Another potential benefit of not using a separate stylet is a reduced rate of intracranial hemorrhage due to a reduced number of trajectory passes. Multiple authors cite a relationship between the number of electrode passes in deep brain stimulation and hemorrhage risk, and one could assume a similar correlation in SEEG.[
The thin profile of NeuroOne electrodes provides an additional technical specification that might reduce hemorrhage risk. With a diameter of 0.8 mm, NeuroOne has a slim profile [
With a learning curve for insertion considered, it would be reasonable to infer small cost savings from the shortened duration of anesthesia if the surgeon fully realizes the potential surgical time benefits of the NeuroOne tapered drill and internal stylet.
Although this series is one of the earliest reports of NeuroOne SEEG electrodes, it includes a very limited number of patients. Due to an overall low complication rate with SEEG, a larger number of electrode insertions would be necessary to compare operative times, technical issues, or hardware complications between these and other electrode models. The same statement could be made about the diagnostic capabilities of this electrode versus other available designs. This text is intended to be a review of some technical specifications of this new SEEG product and not a lengthy review of the indications for SEEG. There are many similarities between currently approved electrode models. The lack of a separate stylet for NeuroOne electrode insertion may prompt the concern of electrode deviation; the risk of this event is unknown and would be elicited with a large volume of consecutive cases. Finally, as the variable of cost is influenced by many factors – insurance, availability, and hospital contractual agreements, it may be difficult to generalize trends.
CONCLUSION
Our initial experience with NeuroOnc SEEG products gives us positive expectations for continued use in epilepsy surgery. Their low profile, variability in contact spacing, and semi-rigid internal stylet design suggest that they can be both versatile and efficient for the surgeon in terms of OR time and cost. There are multiple similarities in design between the existing electrode companies, which will make studying some differences challenging, whether this may be hardware complications, successful use in monitoring data, or risk of complications in a procedure with already low complication rates.
Author contributions
NW: Conceptualization, data curation, formal analysis, writing of original draft, review, and editing; AH: Conceptualization, review, and editing; SV: Conceptualization, formal analysis, review and editing, supervision.
Ethical approval
The research/study was approved by the Institutional Review Board at the University of California Irvine, number 3656, dated 10/01/2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Video available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We would like to recognize all surgical device company representatives who have worked at UCI in their efforts to provide accurate information and assist with patient care.
References
1. Cardinale F, Cossu M, Castana L, Casaceli G, Schiariti MP, Miserocchi A. Stereoelectroencephalography: Surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery. 2013. 72: 353-66
2. De Almeida AN, Olivier A, Quesney F, Dubeau F, Savard G, Andermann F. Efficacy of and morbidity associated with stereoelectroencephalography using computerized tomography-or magnetic resonance imaging-guided electrode implantation. J Neurosurg. 2006. 104: 483-7
3. Faraji AH, Remick M, Abel TJ. Contributions of robotics to the safety and efficacy of invasive monitoring with stereoelectroencephalography. Front Neurol. 2020. 16: 570010
4. , editors. FDA approval of neuroone electrodes. K211367.pdf. 2021. p.
5. Gomes FC, Larcipretti AL, Nger G, Dagostin CS, UdomaUdofa OC, Pontes JP. Robot-assisted vs. manually guided stereoelectroencephalography for refractory epilepsy: A systematic review and meta-analysis. Neurosurg Rev. 2023. 46: 102
6. González-Martínez J, Bulacio J, Thompson S, Gale J, Smithason S, Najm I. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery. 2016. 78: 169-80
7. Lee SJ, Lee PS, Faraji AH, Richardson RM, Kokkinos V. Implantation accuracy and operative variables in robot-assisted stereoelectroencephalography. J Neurosurg. 2023. 139: 1598-603
8. McGovern R, Ruggieri P, Bulacio J, Najm I, Bingaman W, Gonzalez-Martinez J. Risk analysis of hemorrhage in stereo-electrocephalography procedures. Epilepsia. 2019. 60: 571-80
9. Mullin J, Smithason S, Gonzalez-Martinez J. Stereo-electro-encephalo-graphy (SEEG) with robotic assistance in the presurgical evaluation of medical refractory epilepsy: A technical note. J Vis Exp. 2016. 112: e53206
10. Mullin JP, Shriver M, Alomar S, Najm I, Bulacio J, Chauvel P. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia. 2016. 57: 386-401
11. Zhang D, Cui X, Zheng J, Zhang S, Wang M, Lu W. Neurosurgical robot-assistant stereoelectroencephalography system: Operability and accuracy. Brain Behav. 2021. 11: e2347
12. Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: A large case series and systematic literature review. J Neurosurg. 2012. 116: 84-94