- Department of Neurosurgery, Tachikawa General Hospital, Nagaoka, Japan
- Department of Neuroradiology, Brain Research Institute, University of Niigata, Niigata, Japan
- Department of Neurosurgery, Takeda General Hospital, Aizuwakamatsu, Japan
- Department of Neurosurgery, Brain Research Institute, University of Niigata, Niigata, Japan
Correspondence Address:
Hiroshi Abe, Department of Neurosurgery, Tachikawa General Hospital, Nagaoka, Japan.
DOI:10.25259/SNI_211_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Toshiharu Nomura1, Kouichirou Okamoto2, Hiroshi Abe1, Hideaki Abe3, Hitoshi Hasegawa4. Early cerebral hypoperfusion with small artery vasospasm and delayed development of cortical T2* hypointense lesions in contrast-induced encephalopathy. 02-May-2025;16:168
How to cite this URL: Toshiharu Nomura1, Kouichirou Okamoto2, Hiroshi Abe1, Hideaki Abe3, Hitoshi Hasegawa4. Early cerebral hypoperfusion with small artery vasospasm and delayed development of cortical T2* hypointense lesions in contrast-induced encephalopathy. 02-May-2025;16:168. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13528
Abstract
BackgroundContrast-induced encephalopathy (CIE) is a rare complication arising from neurotoxicity caused by iodinated contrast agents. Its clinical presentation closely resembles that of acute stroke, which can occur following endovascular procedures. Distinguishing these two conditions is essential for proper patient management but is challenging based on clinical symptoms alone. While characteristic computed tomography findings for CIE and magnetic resonance imaging (MRI) findings for acute stroke are well established, early MRI findings of CIE – particularly in magnetic resonance angiography (MRA) and perfusion imaging (MRP) – remain underrecognized. In addition, delayed cortical hypointensities on T2*-weighted imaging (T2*WI) in chronic-stage cases have not been previously reported.
Case DescriptionA 78-year-old woman developed CIE immediately following endovascular coil embolization for an unruptured cerebral aneurysm. Small artery vasospasms and hypoperfusion were identified on MRA and MRP, respectively, in the affected hemisphere 6 h postprocedure and resolved a day before symptom improvement. Ten months later, asymptomatic, punctate, and short linear cortical hypointensities appeared on T2*WI, suggesting microhemorrhages.
ConclusionEarly transient small artery vasospasms and hypoperfusion on neuroimaging are indicative of CIE and likely contribute to the neurological symptoms of this condition. Persistent blood–brain barrier dysfunction may underlie the delayed development of cortical hypointensities on T2*WI, seen in chronic-stage CIE cases.
Keywords: Blood–brain barrier dysfunction, Contrast-induced encephalopathy, Hypoperfusion, Magnetic resonance imaging, Microhemorrhage, Vasospasm
INTRODUCTION
Contrast-induced encephalopathy (CIE) is a rare complication resulting from the neurotoxicity of iodinated contrast agents (iCAs).[
In this report, we present serial magnetic resonance (MR) findings in a patient who developed CIE immediately following endovascular coil embolization for an unruptured cerebral aneurysm. During the acute phase of CIE, cerebral small artery vasospasms, and decreased cerebral perfusion were observed in the affected hemisphere using MR angiography (MRA) and MR perfusion (MRP) imaging with arterial spin labeling (ASL), respectively. These imaging abnormalities were transient, resolving before any noticeable improvement in neurological symptoms. On T2*-weighted imaging (T2*WI) obtained 10 months after the procedure, several punctate hypointensities, suggestive of microhemorrhages, were noted in the affected cerebral cortex. These microhemorrhages were asymptomatic. Prolonged blood–brain barrier (BBB) dysfunction has been proposed as a persistent pathomechanism contributing to the acute phase of CIE.
CASE DESCRIPTION
A 78-year-old left-handed woman presented with a history of hypertension presented to a local hospital with dizziness. While head MRI revealed no lesions associated with the dizziness, multiple cerebral aneurysms were incidentally detected on MRA in the parasellar portion of the right internal carotid artery (ICA), the A2–A3 bifurcation of the right anterior cerebral artery (ACA), and the M1– M2 bifurcation of the left middle cerebral artery (MCA) [
Figure 1:
Neuroimaging (MRI and MRA) on admission. (a) DWI, (b) FLAIR, (c) MRA. (a) No hyperintense lesions are observed on DWI. (b) Several small hyperintense lesions are visible in the cerebral white matter on FLAIR images. (c) Multiple small aneurysms are noted at the parasellar portion of the right internal carotid artery (ICA), the right A2–A3 bifurcation, and the left M1–M2 bifurcation (arrowheads). DWI: Diffusion-weighted imaging, FLAIR: Fluid-attenuated inversion recovery, ICA: Internal carotid artery, MRA: Magnetic resonance angiography, MRI: Magnetic resonance imaging.
Upon admission, the patient was alert and conscious. Physical and neurological examinations revealed no abnormalities. Her height was 148 cm, weight 42 kg, body temperature 36.6°C, blood pressure 138/85 mmHg, pulse 84/min, and regular. Blood chemistry results were within normal limits: blood urea nitrogen, 18.6 mg/dL; creatinine, 0.70 mg/dL; estimated glomerular filtration rate, 61 mL/min; sodium, 140 mmol/L; potassium, 4.4 mmol/L; chloride, 105 mmol/L; and creatine kinase, 66 U/L.
Three-dimensional rotational digital subtraction angiography (3D-DSA) showed no stenosis of the cervical and intracranial arteries and confirmed the presence of multiple saccular aneurysms. The sizes of the aneurysms were as follows: right parasellar ICA 3.0 × 2.8 × 2.8 mm, left M2 superior trunk 3.2 × 4.6 × 2.4 mm, left M2 inferior trunk 3.1 × 3.6 × 2.6 mm, and right A2–A3 bifurcation 4.2 × 4.6 × 4.3 mm. The iCA used was iomeprol (Iomeron®,
Bracco, Milano, Italy), a low-osmolar, nonionic agent heated to 37°C, with a total volume of 110 ml. Nausea occurred during the examination but resolved spontaneously within 10 h. Based on the 3D-DSA findings, coil embolization was planned for the largest right A2–A3 bifurcation aneurysm. The patient began oral clopidogrel 75 mg and aspirin 100 mg once daily, starting 6 days and 2 days before the endovascular procedure. Oral prednisolone 30 mg was prescribed the night before the procedure to minimize potential side effects from the contrast agent.
Under general anesthesia, an 8-Fr sheath was inserted through the right femoral artery. After introducing the 8-Fr ROADMASTER TH (NIPRO, Osaka, Japan) into the right cervical ICA, a 6-Fr SOFIASELECT (TERUMO, Tokyo, Japan) was positioned in the cavernous portion (C3 segment) of the right ICA as an intermediate catheter. Excelsior SL10 (Stryker, Fremont, CA, USA) and Phenom17 (Medtronic, Dublin, Ireland) catheters were advanced into the right A2–A3 bifurcation aneurysm and coil embolization was successfully performed using a double-catheter technique with three coils: Target XL 360 soft mini 4 mm × 12 cm (Stryker, Fremont, CA, USA), Axium Prime 3D 3 mm × 8 cm (Medtronic, Dublin, Ireland), and i-ED coil complex 10∞SS 1.5 mm × 3 cm (Kaneka Medics, Osaka, Japan). The procedure lasted 1 h and 5 mins. No blood stagnation was observed throughout, and postembolization DSA showed no vascular occlusion or vasospasms. In addition, 90 mL of Iopamidol (Iopamiron®, Bayer, Nordrhein-Westfalen, Germany), another low-osmolar, nonionic iCA, was used and heated to 37°C.
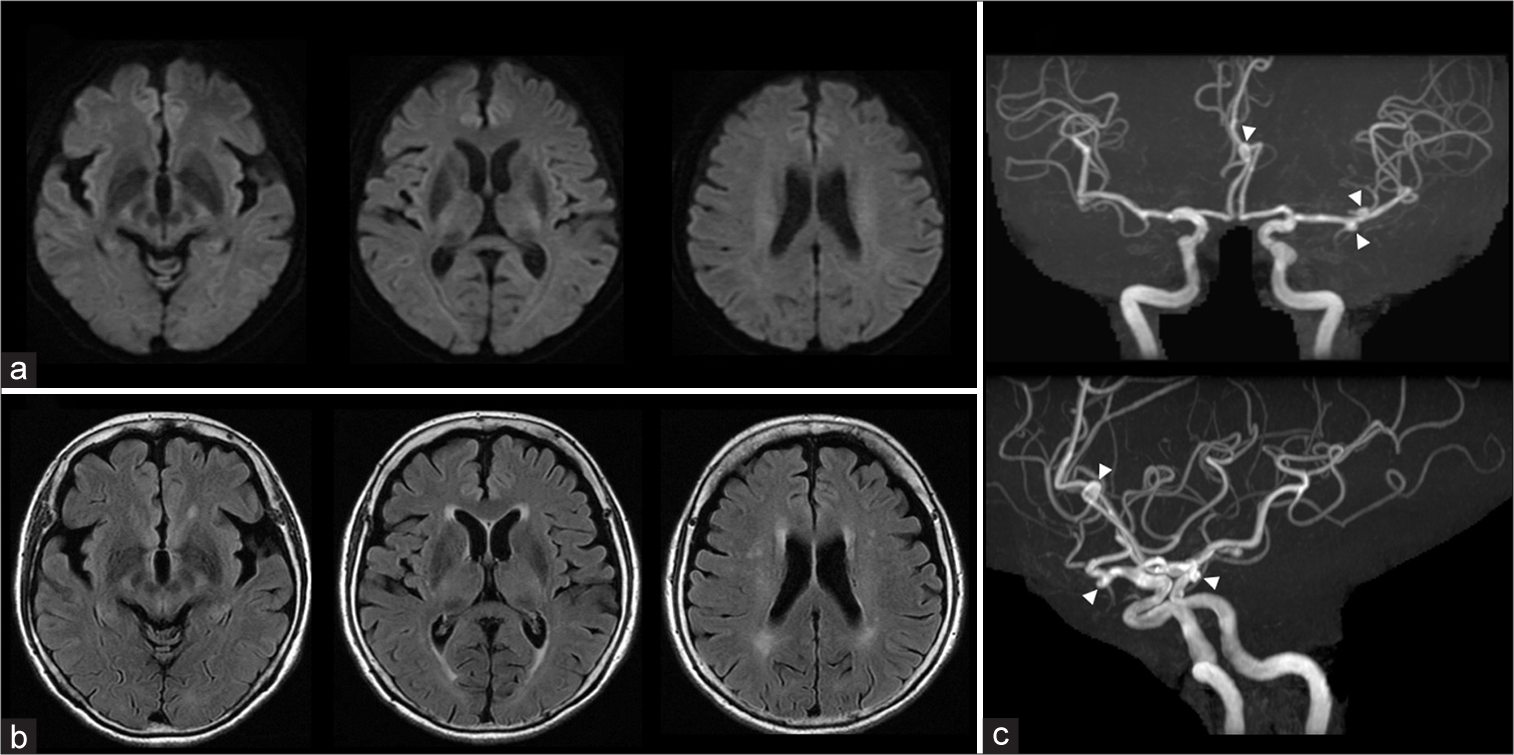
Shortly after awakening from general anesthesia, the patient developed left hemiplegia, aphasia, and pain in the right frontal region. Brain MRI obtained 1 h after the procedure showed no acute ischemic or hemorrhagic changes on diffusion-weighted imaging (DWI) [
Figure 2:
Neuroimaging (MRI and MRA) 1 h after the procedure. (a) DWI, (b) FLAIR, (c) MRA. (a and b) No new lesions are observed on MRI. (c) Delineation of the peripheral branches of the right MCA and ACA is slightly diminished on MRA. DWI: Diffusion-weighted imaging, FLAIR: Fluid-attenuated inversion recovery, MCA: Middle cerebral artery, ACA: Anterior cerebral artery, MRA: Magnetic resonance angiography, MRI: Magnetic resonance imaging.
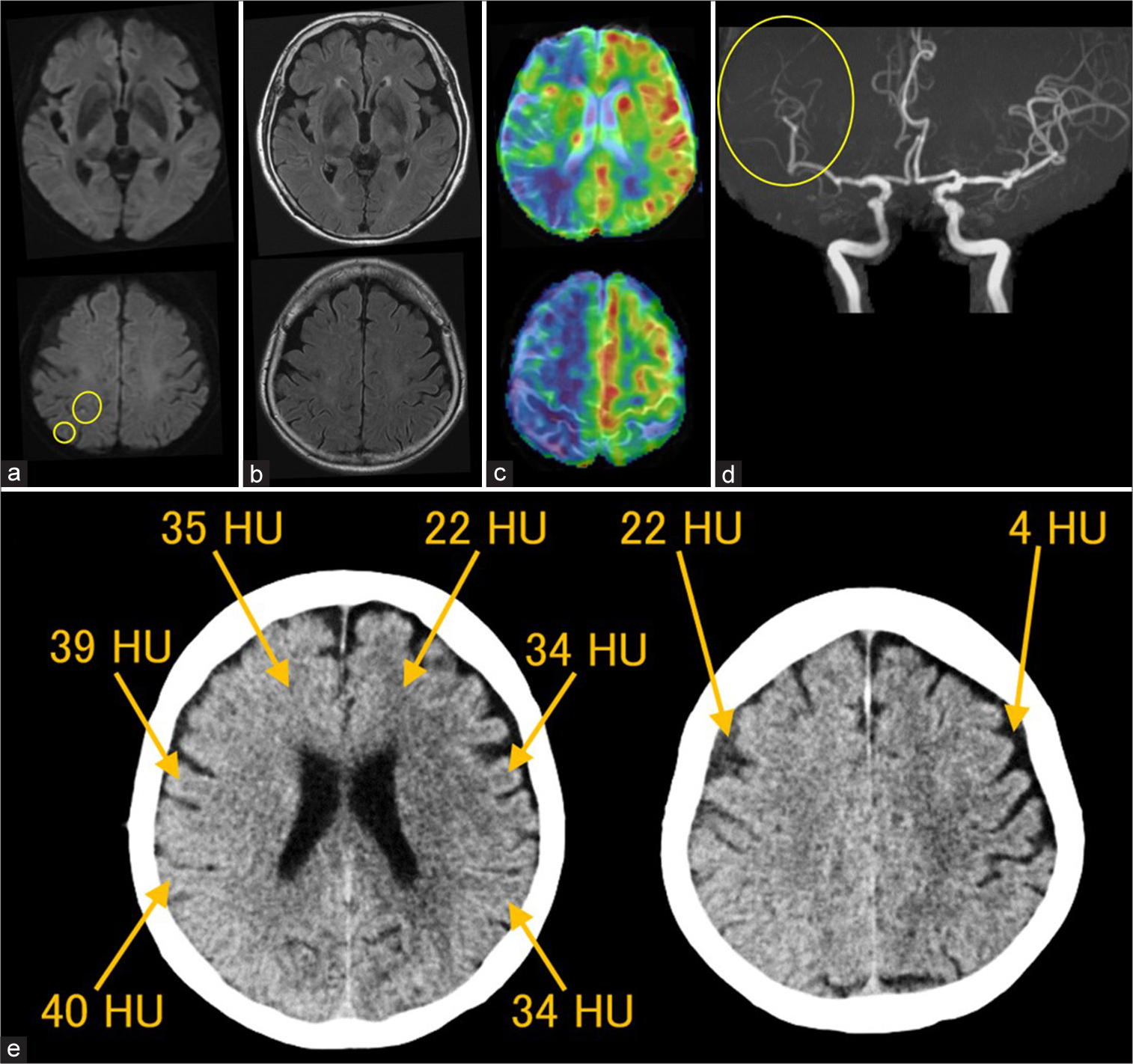
Figure 3:
Neuroimaging (MRI, MRP, MRA, and CT) 6 h after the procedure. (a) DWI, (b) FLAIR, (c) MRP, (d) MRA, (e) CT. (a) DWI reveals multiple punctate cortical hyperintense lesions in the right MCA distribution (circles). (b) No additional hyperintense lesions are seen on FLAIR images. (c) MRP with ASL (PLD 1,525 ms) shows hypoperfusion in the right cerebral hemisphere. (d) MRA reveals small artery vasospasms in the peripheral branches of the right MCA (a circle) and ACA. (e) CT obtained 6.5 h postprocedure shows decreased gray/white matter contrast and diffuse increased attenuation in the right MCA and ACA territories, as well as in the right cerebral subarachnoid spaces. The CT values are as follows: right cerebral cortex, 39–40 HU; left cerebral cortex, 34 HU; right frontal white matter, 35 HU; left frontal white matter, 22 HU; right frontal subarachnoid space, 22 HU; left frontal subarachnoid space, 4 HU. ACA: Anterior cerebral artery, ASL: Arterial spin labeling, CT: Computed tomography, DWI: Diffusion-weighted imaging, FLAIR: Fluid-attenuated inversion recovery, HU: Hounsfield unit, MRA: Magnetic resonance angiography, MCA: Middle cerebral artery, MRP: Magnetic resonance perfusion imaging, PLD: Postlabeling delay.
MRP imaging using ASL (postlabeling delay [PLD] 1,525 ms) indicated right cerebral hypoperfusion [
Based on these findings, CIE was suspected. A subsequent head computed tomography (CT) scan showed decreased gray/white matter contrast, diffusely increased attenuation in the cerebral cortex, subcortical white matter, and subarachnoid spaces within the right MCA territory [
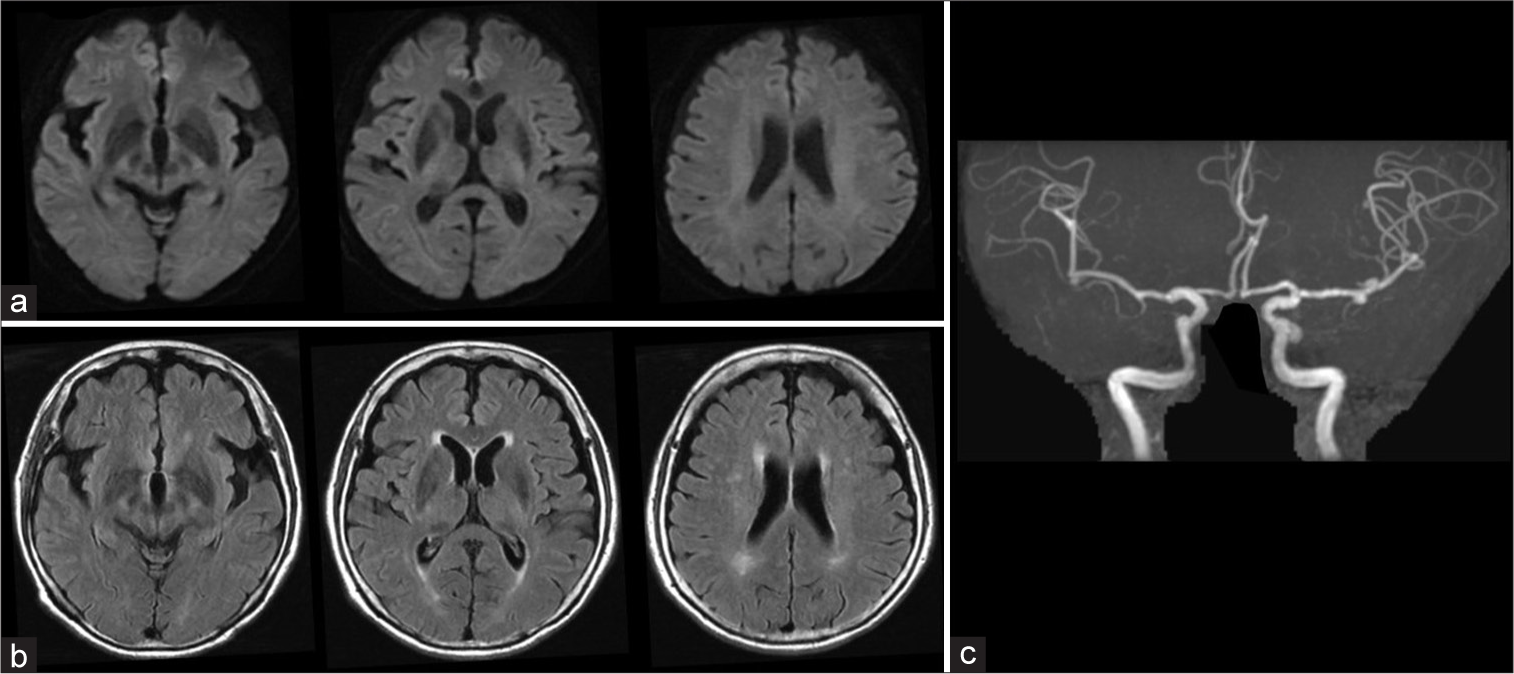
The following day, additional punctate hyperintense lesions with restricted diffusion were observed in the cortices and subcortical regions of the right MCA territory on DWI, although the vasospasms and right cerebral hypoperfusion had normalized [
Figure 4:
Neuroimaging (MRI, MRP, and MRA) on the day after the procedure. (a) DWI, (b) FLAIR, (c) MRP, (d) MRA. (a) Multiple punctate hyperintense lesions are detected in the right MCA territory on DWI (circles). (b) FLAIR images remain unchanged. (c) Hypoperfusion in the right cerebral hemisphere is resolved on the MRP with ASL (PLD 1,525 ms). (d) Small artery vasospasms in the right MCA and ACA are no longer visible on MRA. ASL: Arterial spin labeling, DWI: Diffusion-weighted imaging, FLAIR: Fluid-attenuated inversion recovery, MCA: Middle cerebral artery, MRA: Magnetic resonance angiography, MRP: Magnetic resonance perfusion imaging, MRI: Magnetic resonance imaging, PLD: Postlabeling delay.
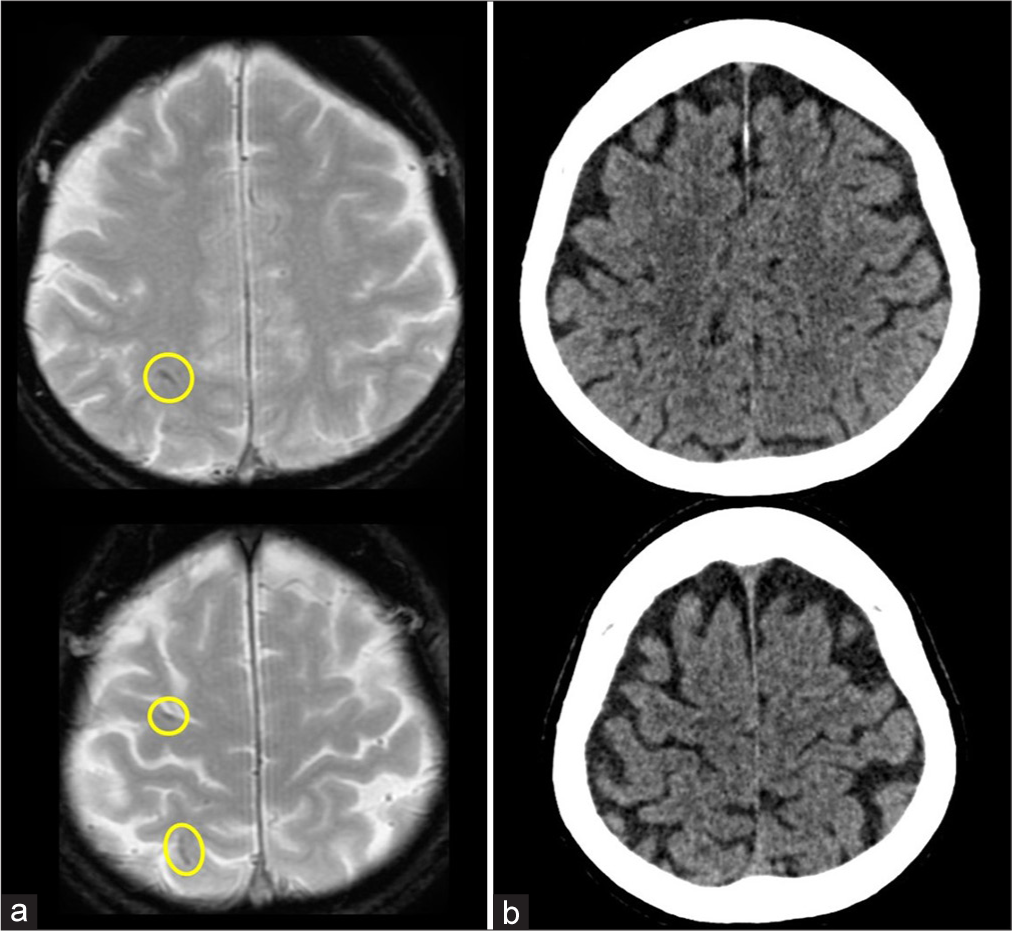
The patient’s clinical course after discharge was uneventful, and no new neurological symptoms developed. The patient did not undergo any further imaging examinations with iCA. However, T2*WI performed 10 months later revealed additional punctate and short, linear hypointense lesions on the right cerebral cortex, distinct from the previous punctate hyperintense lesions seen on DWI [
Figure 5:
Neuroimaging (MRI and CT) 10 months after the procedure. (a) T2*WI, (b) CT with 1-mm slice thickness. (a) A few additional punctate and short linear hypointense lesions are observed in different regions of the right cerebral cortex compared to the previous hyperintense lesions on DWI (circles). No similar lesions are seen outside the right MCA territory. (b) No corresponding hyperattenuation is seen on CT, suggesting no deposition of iodinated contrast agent, calcification, or acute hemorrhage. CT: Computed tomography, DWI, Diffusion-weighted imaging, MCA: Middle cerebral artery, MRI: Magnetic resonance imaging, T2*WI: T2-star-weighted imaging.
DISCUSSION
Over 2,000 patients have been treated endovascularly for cerebral aneurysms at our hospital, and we have made efforts to minimize the use of intra-arterial iCA in these procedures. To date, we have not encountered any cases of CIE during diagnostic cerebral angiography or endovascular treatment, with the exception of the current patient. This is our first experienced case of symptomatic CIE following coil embolization of an unruptured cerebral aneurysm using an intermediate catheter.
Our patient developed right frontal headache, left hemiplegia, aphasia, left neglect, and impaired consciousness after coil embolization of an unruptured right ACA aneurysm. Although acute ischemic or hemorrhagic complications were initially suspected, no hyperintense or hypointense lesions were identified on the initial DWI obtained 1 h after the procedure. However, repeat neuroimaging, performed 6 h later when the patient’s symptoms had worsened, revealed several punctate cortical DWI hyperintensities, vasospasm of the right MCA and ACA distal branches, and cerebral hypoperfusion in the area where the iCA was injected. Subsequent head CT showed increased CT values in the brain parenchyma and subarachnoid spaces of the affected right hemisphere, confirming the diagnosis of CIE. DWI performed the following day revealed additional small hyperintense lesions in the right cerebral cortex. Remarkably, the resolution of vasospasms and hypoperfusion preceded symptomatic improvement. After her symptoms improved, the patient had an uneventful clinical course. A T2*WI scan performed 10 months later showed several punctate or short linear hypointensities, but these were asymptomatic.
CIE is a pathological condition in which the BBB is disrupted by the high osmotic pressure and/or chemical toxicity of the iCA in the cerebral arteries. Neurological symptoms are caused by neurotoxicity when iCA leaks from the cerebral vessels into the brain parenchyma through the compromised BBB.[
The diagnostic criteria for CIE, as proposed by Chu et al., include clinical symptoms distinct from the primary disease and the appearance of hyperattenuation in the brain parenchyma and subarachnoid spaces on CT, excluding hemorrhage or infarction.[
In a study of 396 cerebral aneurysm coil embolizations, Fuga et al. identified iCA injection from the intracranial arteries as an independent risk factor for CIE.[
Previously reported MRI findings of CIE have shown diffusely increased signal intensity and swelling of the cerebral cortex on T2WI, fluid-attenuated inversion recovery (FLAIR) imaging, and DWI without diffusion restriction.[
Posterior reversible encephalopathy syndrome (PRES) can develop after coronary angiography and cerebrovascular interventions using iCA. BBB dysfunction is believed to be a common pathophysiological factor in both CIE and PRES.[
iCAs cause shortening of T1 and T2 signals on MRI, although no signal intensity changes are detected on T1WI, T2WI, or T2*WI with 1.5-T and 3.0-T MR scanners used clinically, as the iCAs are diluted in blood or brain parenchyma.[
Symptoms of CIE are usually transient and reversible. However, sequelae of varying degrees have been documented in cases of CIE[
CONCLUSION
CIE should be considered when a patient develops disturbances in consciousness and/or focal neurological deficits during or after selective catheter cerebral angiography, with or without endovascular intervention. Early diagnosis is essential to initiate appropriate therapies based on the pathophysiology of CIE in the acute phase. In addition to characteristic CT findings, a combination of early MR findings, such as hemispheric hypoperfusion and small artery vasospasms, is valuable for diagnosing CIE and understanding its pathophysiology. The increased use of intermediate catheters may be a new risk factor for CIE, and attention should be given to catheter positioning during iCA injection. Although most patients with CIE recover within several days, asymptomatic punctate hypointensities resembling cerebral microhemorrhages may appear on T2*WI even after the acute phase. Long-term follow-up of such patients is necessary to investigate the pathophysiology of CIE further.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alp BN, Bozbuğa N, Tuncer MA, Yakut C. Transient cortical blindness after coronary angiography. J Int Med Res. 2009. 37: 1246-51
2. Chu YT, Lee KP, Chen CH, Sung PS, Lin YH, Lee CW. Contrast-induced encephalopathy after endovascular thrombectomy for acute ischemic stroke. Stroke. 2020. 51: 3756-9
3. Cristaldi PM, Polistena A, Patassini M, De Laurentis C, Giussani C, Remida P. Contrast-induced encephalopathy and permanent neurological deficit: A case report and literature review. Surg Neurol Int. 2021. 12: 273
4. De Falco A, De Simone M, D’Onofrio F, Spitaleri D, De Falco FA. Posterior reversible encephalopathy syndrome overlapping contrast-induced encephalopathy after coronary angiography. Neurol Sci. 2019. 40: 1951-3
5. Fuga M, Tanaka T, Tachi R, Yamana S, Irie K, Kajiwara I. Contrast injection from an intermediate catheter placed in an intradural artery is associated with contrast-induced encephalopathy following neurointervention. AJNR Am J Neuroradiol. 2023. 44: 1057-63
6. Giraldo EA, Fugate JE, Rabinstein AA, Lanzino G, Wijdicks EF. Posterior reversible encephalopathy syndrome associated with hemodynamic augmentation in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011. 14: 427-32
7. González IA, Tapia C, Hernández-Luis C, San Román JA. Contrast neurotoxicity following percutaneous revascularization. Rev Esp Cardiol. 2008. 61: 894-6
8. Kamimura T, Nakamori M, Imamura E, Hayashi Y, Matsushima H, Mizoue T. Low-dose contrast-induced encephalopathy during diagnostic cerebral angiography. Intern Med. 2021. 60: 629-33
9. Kawasaki T, Hayase M, Miyakoshi A, Taki J, Nakamura T, Hatano T. Two cases of symptomatic contrast-induced encephalopathy after coil embolization of unruptured cerebral aneurysm. J Neurendovasc Ther. 2015. 9: 96-102
10. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology. 1989. 39: 567-71
11. Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv Neuroradiol. 2012. 18: 33-41
12. Li J, Qi G, Zhang H, Chen G, Wang S, Yan M. Contrast-induced encephalopathy mimicking stroke after a second cerebral DSA: An unusual case report. BMC Neurol. 2021. 21: 430
13. Merchut MP, Richie B. Transient visuospatial disorder from angiographic contrast. Arch Neurol. 2002. 59: 851-4
14. Nagamine Y, Hayashi T, Kakehi Y, Yamane F, Ishihara S, Uchino A. Contrast-induced encephalopathy after coil embolization of an unruptured internal carotid artery aneurysm. Intern Med. 2014. 53: 2133-8
15. Nikoubashman O, Jablawi F, Dekeyzer S, Oros-Peusquens AM, Abbas Z, Lindemeyer J. MRI appearance of intracerebral iodinated contrast agents: Is it possible to distinguish extravasated contrast agent from hemorrhage?. AJNR Am J Neuroradiol. 2016. 37: 1418-21
16. Romano DG, Frauenfelder G, Locatelli G, Panza MP, Siani A, Tartaglione S. Arterial spin labeling magnetic resonance imaging to diagnose contrast-induced vasospasm after intracranial stent embolization. World Neurosurg. 2019. 126: 341-5
17. Saigal G, Bhatia R, Bhatia S, Wakhloo AK. MR findings of cortical blindness following cerebral angiography: Is this entity related to posterior reversible leukoencephalopathy?. AJNR Am J Neuroradiol. 2004. 25: 252-6
18. Silverman SM, Bergman PS, Bender MB. The dynamics of transient cerebral blindness. Report of nine episodes following vertebral angiography. Arch Neurol. 1961. 4: 333-48
19. Skolarus LE, Gemmete JJ, Braley T, Morgenstern LB, Pandey A. Abnormal white matter changes after cerebral aneurysm treatment with polyglycolic-polylactic acid coils. World Neurosurg. 2010. 74: 640-4
20. Spina R, Simon N, Markus R, Muller DW, Kathir K. Contrast-induced encephalopathy following cardiac catheterization. Catheter Cardiovasc Interv. 2017. 90: 257-68
21. Zwicker JC, Sila CA. MRI findings in a case of transient cortical blindness after cardiac catheterization. Catheter Cardiovasc Interv. 2002. 57: 47-9