- Department of Neurosurgery, Rawalpindi Medical University, Rawalpindi, Pakistan
- Department of Neurosurgery, Liaquat National Hospital, Karachi, Pakistan
- Department of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
- Department of Neurosurgery, Rehman Medical Institute Peshawar, Peshawar, Pakistan
- Department of Neurosurgery, Sohail Trust Hospital, Karachi, Pakistan
Correspondence Address:
Saad Akhtar Khan, Department of Neurosurgery, Liaquat National Hospital, Karachi, Sindh, Pakistan.
DOI:10.25259/SNI_737_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hamza Khan1, Abdul Basit Sangah2, Roua Nasir3, Saad Akhtar Khan2, Shazia Saleem Shaikh2, Ikhlas Ahmed2, Mohad Kamran Abbasi4, Asma Ahmed4, Dua Siddiqui2, Syeda Ayesha Hussain4, Naveed Zaman Akhunzada4, Oswin Godfrey5. Efficacy of radiosurgery with and without angioembolization: A subgroup analysis of effectiveness in ruptured versus unruptured arteriovenous malformations – An updated systematic review and meta-analysis. 20-Dec-2024;15:467
How to cite this URL: Hamza Khan1, Abdul Basit Sangah2, Roua Nasir3, Saad Akhtar Khan2, Shazia Saleem Shaikh2, Ikhlas Ahmed2, Mohad Kamran Abbasi4, Asma Ahmed4, Dua Siddiqui2, Syeda Ayesha Hussain4, Naveed Zaman Akhunzada4, Oswin Godfrey5. Efficacy of radiosurgery with and without angioembolization: A subgroup analysis of effectiveness in ruptured versus unruptured arteriovenous malformations – An updated systematic review and meta-analysis. 20-Dec-2024;15:467. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13298
Abstract
Background: Congenital arterial defects such as cerebral arteriovenous malformations (AVMs) increase brain bleeding risk. Conservative therapy, microsurgical removal, percutaneous embolization, stereotactic radiosurgery (SRS), or a combination may treat this serious disease. This study compares angioembolization with SRS to SRS alone in ruptured or unruptured brain ateriovenous malformations (BAVM) patients.
Methods: We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations for this study. Until September 2023, PubMed/Medline, Cochrane, and Clinicaltrials.gov were searched for literature. English-language studies comparing SRS alone to embolization with SRS on ruptured or non-ruptured AVMs that could not be operated on were considered. The Newcastle–Ottawa Scale assessed research study quality.
Results: Results included 46 studies with a total of 7077 participants. There was a greater obliteration rate in the SRS-only group (60.4%) than in the embolization plus SRS group (49.73%). Particularly in the SRS-only group, ruptured AVMs showed a noticeably greater obliteration rate than unruptured AVMs (P = 0.002). However, no notable differences were found in hemorrhagic events or radiation-induced changes between the two groups; however, the SRS-only group had a slightly greater, yet not statistically significant, mortality rate.
Conclusion: Our data showed that ruptured brain AVMs had a much greater obliteration rate than unruptured ones, mostly due to SRS alone, without embolization. The aggregated data showed no significant changes, whereas SRS alone decreased radiation-induced alterations and hemorrhagic rates but with increased mortality. SRS alone may have a higher risk-to-reward ratio for nidus obliteration in ruptured brain AVM patients, so it should be used without embolization, although more research is needed to determine the effects of immediate and late complications.
Keywords: Angioembolization, Cerebral arteriovenous malformations, Hemorrhagic events, Obliteration rate, Radiation-induced changes, Stereotactic radiosurgery
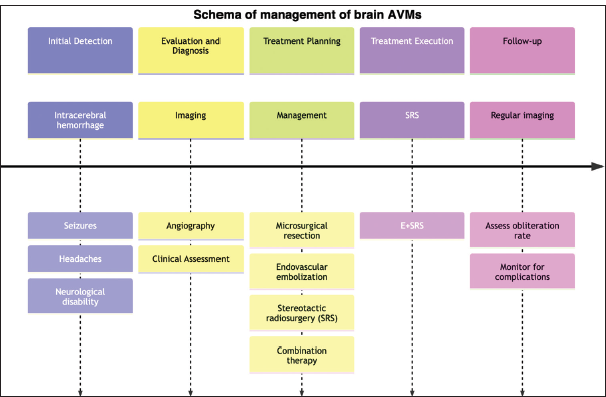
INTRODUCTION
AVMs, or cerebral arteriovenous malformations, are genetic anomalies that are characterized by aberrant arteries and veins without a capillary bed in between.[
Endovascular embolization is frequently utilized as a preoperative adjunctive treatment option for major AVMs and can also serve as the primary therapy for smaller AVMs that are difficult to treat surgically.[
With this review, we aim to assess the most up-to-date data to determine the efficacy of angioembolization as an additional therapy for SRS in in-operable AVMs. We also aim to conduct a subgroup analysis to evaluate the obliteration rate in ruptured and unruptured AVMs using “SRS only” or “embolization + SRS” treatment modalities, the segregation of which has not been a part of previous work such as by Chang et al.[
METHODOLOGY
Search strategy
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations in our research.[
Inclusion and exclusion criteria
The parameters used as inclusion criteria for our study are as follows: (1) English-language publications, (2) randomized controlled trials (RCTs), quasi-experimental studies, cohort studies, retrospective cohort studies, (3) patients diagnosed with inoperable ruptured or unruptured AVMs, (4) studies conducted within the past 15 years, and (5) data comparing the use of SRS plus embolization versus SRS alone in patients with ruptured and/or unruptured AVMs. To reduce bias and maintain the reliability of the study, we excluded studies with the following parameters: (1) studies with arteriovenous fistulas, (2) non-comparative studies, qualitative studies (e.g., case series and case reports), (3) studies involving participants without a diagnosis of in-operable ruptured or unruptured AVMs, (4) studies not published in peer-reviewed journals (conference papers and unpublished data), (5) studies with ambiguous data regarding obliteration rates in the embolization + SRS group versus only SRS group, (6) studies evaluating non-intracranial AVM’s, and (7) trials with a high risk of bias, as evaluated by the Newcastle Ottawa scale (NOS).
Data extraction and quality assessment
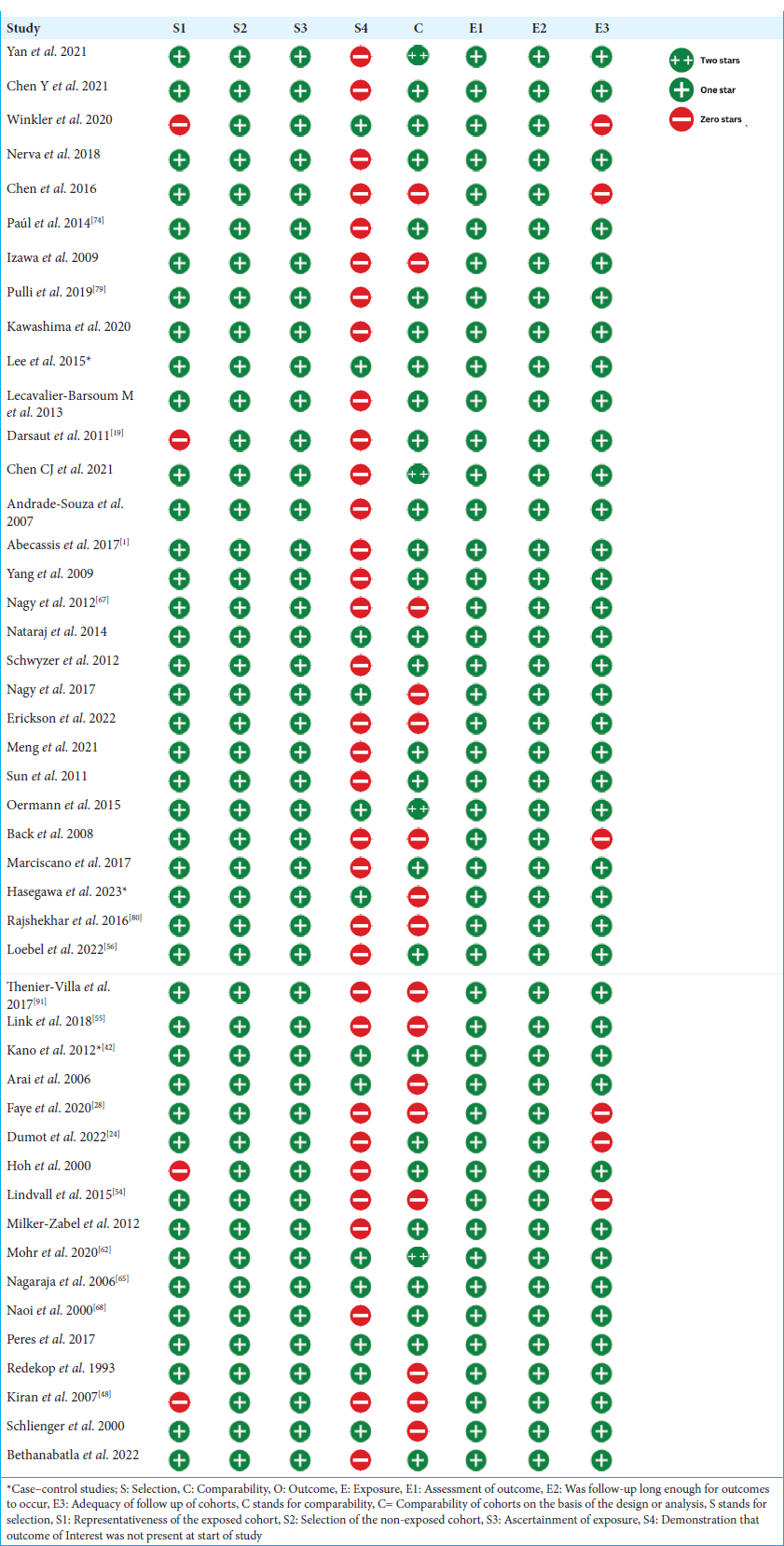
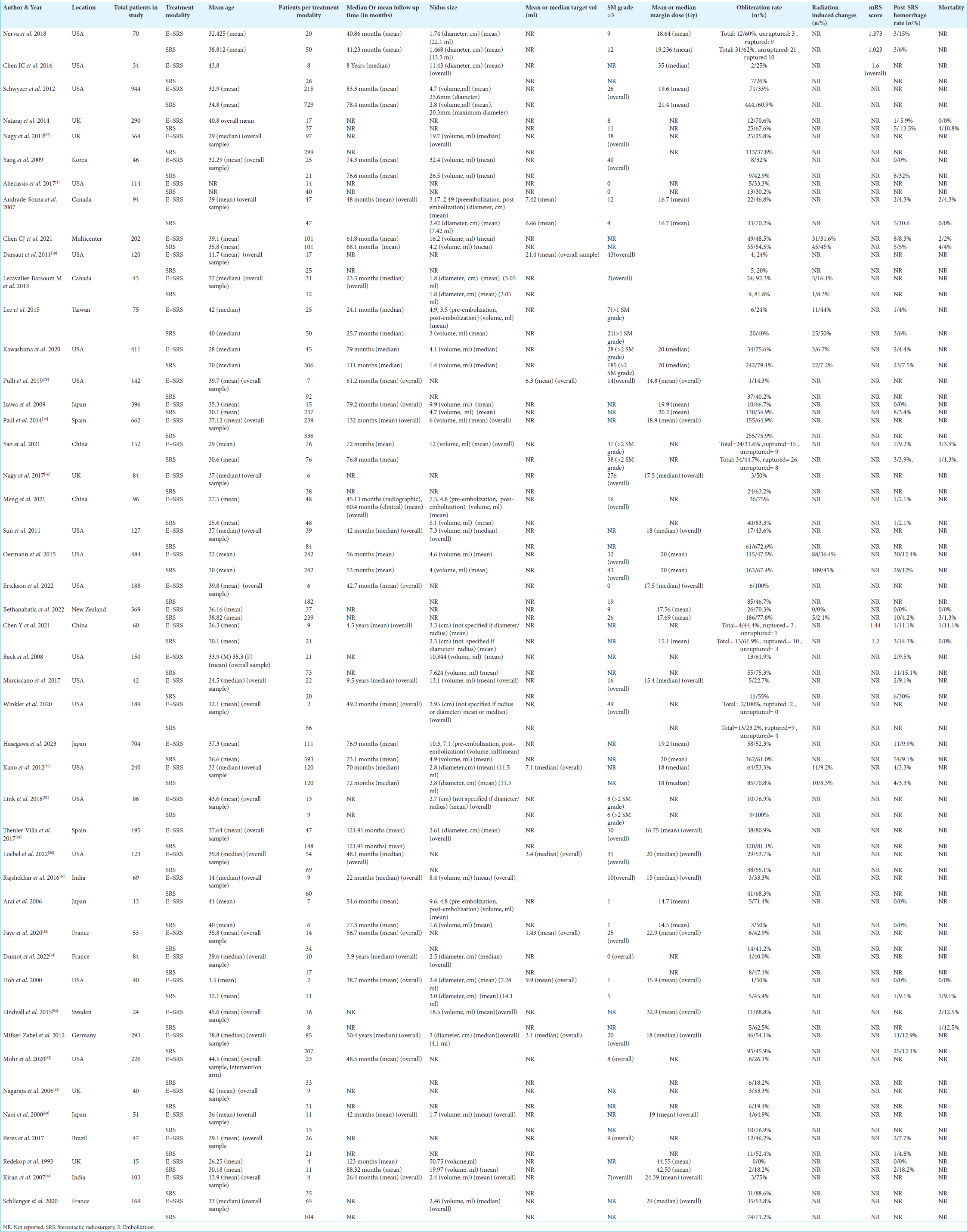
A systemic search strategy was used to obtain reliable results from relevant research databases. Relevant articles were uploaded to Rayyan.ai for screening purposes. Duplicate studies were identified and removed. The remainder of the studies were screened using a two-step process. Originally, articles were assessed by scrutinizing the titles and abstracts of the research papers. Studies that did not fulfill the selection criteria were not included in the study. In the second phase of the screening process, a comprehensive examination was conducted of the complete texts of the remaining articles to determine their conformity with the selection criteria. The pertinent data from the chosen articles were extracted utilizing an Excel spreadsheet. The selected studies were used to gather demographic data, which included information such as the author, year of publication, location of the study, study period, study design, sample size, follow-up duration, mean or median age, and whether the presentation was hemorrhagic or non-hemorrhagic. The study collected data on numerous variables and outcomes, such as the average or median margin dosage, rate of AVM obliteration, occurrence of post-SRS hemorrhage, radiation-induced changes (RICs), and death. The quality of the selected studies was evaluated using the NOS Observational Cohort and Case-Control Studies.[
Statistical analysis
Statistics were done using RevMan 5.4 by Cochrane Library. The cumulative impact for all secondary outcomes as well as the odds ratio (OR) for each study was determined using the Mantel–Haenszel model. Heterogeneity was evaluated using I2 and Chi-square test statistics. Heterogeneity was identified when the Chi-squared test statistic reached a significance level of 10% (P < 0.10). In addition, heterogeneity levels were categorized as low if the I2 value was <40%, substantial if it was >50%, and considerable if it exceeded 75%.[
RESULTS
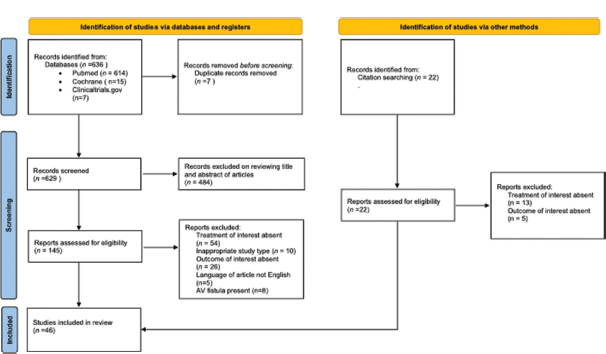
Six hundred and thirty-six potential articles were found using our search method; 484 of these articles were eliminated during the preliminary screening and duplication phase because they did not meet the predetermined inclusion and exclusion criteria [
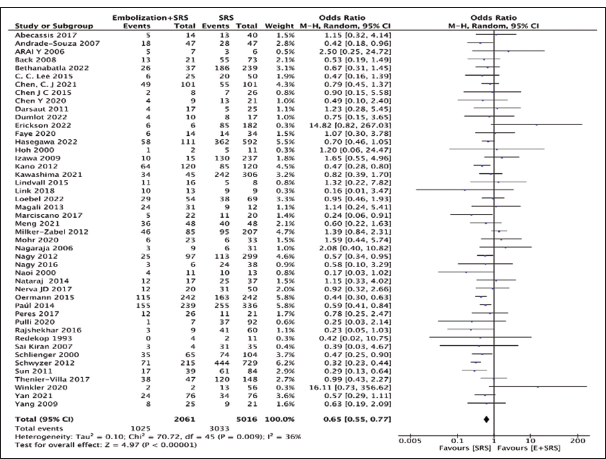
Obliteration rate
Our analysis compared 46 studies to observe obliteration rates in either the “embolization + SRS” group (n = 2061) or the “SRS only” group (n = 5016). The “SRS only” group had a higher obliteration rate (60.4%, n = 3033) compared to the “embolization + SRS” group (49.73%, n = 1025) with a pooled OR of 0.65, 95% CI: 0.55–0.77, 95% CI, P < 0.00001, as shown in
Type of presentation: Ruptured or unruptured brain AVMs
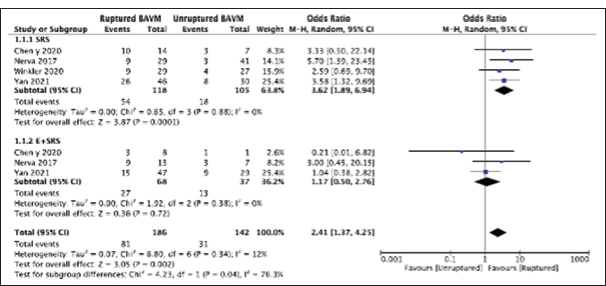
We further assessed differences in obliteration rates based on the type of presentation, either ruptured or unruptured cases of brain AVMs. For our analysis, we included 186 cases for ruptured brain AVMs and 142 for unruptured brain AVMs. Overall, irrespective of the treatment modality of choice, the ruptured brain AVMs (81/186) had a better obliteration rate than unruptured brain AVMs (31/142) with a pooled OR of 2.41, 95% CI: 1.37–4.24, P = 0.002, as shown in
Figure 5:
Forest plot comparing obliteration rates based on type of presentation (unruptured or ruptured brain arteriovenous malformations) and type of intervention (“Stereotactic radiosurgery [SRS] only” or “embolization + SRS”), E: Embolization, BAVM: Brain Arteriovenous malformation, M-H: Mantal-Haenszel, CI: Confidence interval.
Based on the subgroup analysis, the “SRS only” treatment modality improved the obliteration rate for ruptured brain AVMs significantly than for the unruptured brain AVMs, with a pooled OR of 3.62, 95% CI: 1.89–6.94, 95% CI, P < 0.0001, as sown in
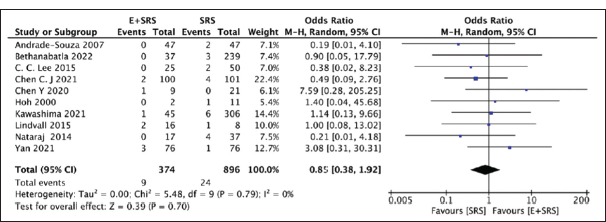
Mortality outcome
Our analysis compared ten studies mentioning mortality outcomes in either the “embolization + SRS” group (9/374) or the “SRS only” group (24/896) and found no significant difference. The “SRS only” group had a slightly higher mortality (2.67%) as compared to the “embolization + SRS” group (2.45%) with a pooled OR of 0.85, 95% CI: 0.38–1.92, and P = 0.7, suggesting the results were non-significant, as shown in
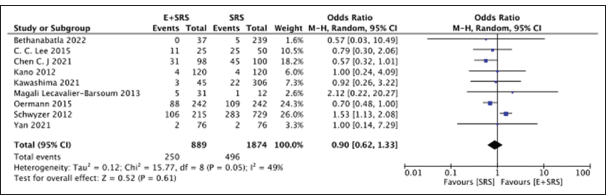
RICs
We further compared nine studies mentioning RICs in either the “embolization + SRS” group (250/889) or the “SRS only” group (496/1874) and found no significant difference. The “SRS only” group had a slightly lower frequency of RICs (28.1%) as compared to the “embolization + SRS” group (26.4%) with a pooled OR of 0.90, 95% CI: 0.62–1.33, P = 0.61, suggesting the results were non-significant, as shown in
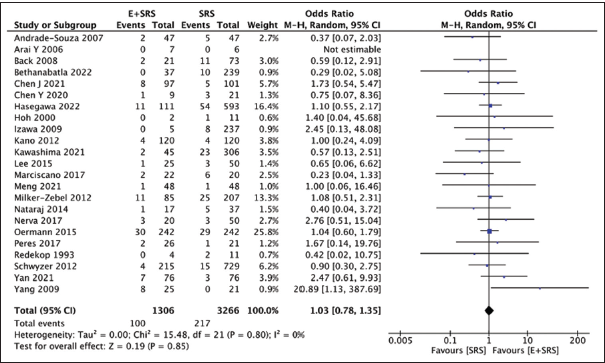
Hemorrhagic events
We additionally compared 23 studies reporting hemorrhagic events in either the “embolization + SRS” group (100/1306) or the “SRS only” group (217/3266) and found no significant difference in the observed rate of hemorrhage in either intervention. There were slightly more hemorrhagic events in the “embolization + SRS” group (7.65%) as compared to the “SRS only” group (6.64%), with a pooled OR of 1.03, 95% CI: 0.78–1.35, P = 0.85, indicating non-significance, as shown in
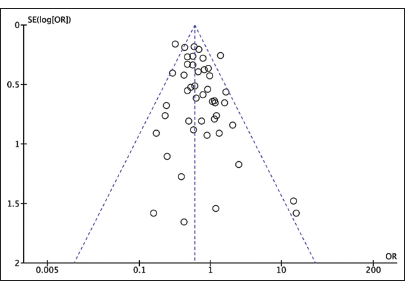
Publication bias
A funnel plot was created using Revman 5.4 to indicate publication bias. Our results indicate a small-study bias, as shown in
DISCUSSION
Brain AVMs are congenital dysplastic groups of dilated blood arteries that bypass the capillary network and have a central nidus connected to an arterial feeder that empties into a vein.[
One well-established method for treating cerebral AVMs is SRS.[
One such variable to consider is the use of different embolizing agents and their effect on clinical outcomes. Studies have highlighted potential causes of complications in different agents. Onyx is a well-researched embolizing material that has certain complications, including incomplete vessel occlusion, as it solidifies from outside inwards, creating a soft inner core that can prevent complete vessel occlusion.[
Obliteration rates as per intervention of choice
Our analysis compared the effectiveness of two treatment methods for brain AVMs: SRS alone and SRS combined with angioembolization. We found that SRS alone resulted in a higher rate of AVM obliteration compared to the combination treatment (60.4% vs. 49.73%). The pooled OR was 0.65, with a 95% CI of 0.55–0.77 and P < 0.00001. In contrast, several other studies[
Obliteration rates as per type of presentation
The presenting status of brain AVMs, ruptured or unruptured, is a potential confounder when analyzing the obliteration rates in patients undergoing different treatment modalities. This finding aligns with other research examining the rates of obliteration in individuals undergoing SRS with either ruptured or unruptured AVM presentations.[
Other outcomes
Hemorrhagic rate
Our findings indicate that there was no statistically significant disparity in the incidence of hemorrhage between the two interventions. This is consistent with prior extensive studies that have been published on the same subject. [
RICs
The pre-SRS embolization may protect patients from RIC;[
Mortality rate
Our investigation showed that the mortality results for the two treatments did not vary statistically significantly. Previous meta-analysis comparing the outcomes between patients undergoing only SRS and those that underwent embolization before SRS found mortality rates to be higher in patients undergoing only SRS, but no information regarding the statistical significance of these results was mentioned.[
Limitations and future implications
The study has limitations, including potential small-study publication bias, heterogeneity in patient demographics, AVM characteristics, treatment techniques, and follow-up durations, and limitations in the design, sample size, and methodological rigor. Furthermore, limited or incomplete data reporting constrains the depth of our analysis. Further research comparing obliteration rates in ruptured and unruptured brain AVMs while controlling for confounding variables, including post-embolization nidus size, treatment modality, intranidal aneurysms, and venous drainage pattern, is required to confirm whether ruptured AVMs have higher obliteration rates than unruptured AVM’s. Future studies with data regarding these factors can help mitigate bias and provide more reliable results.
CONCLUSION
From data from 46 studies, with 7077 patients with brain AVMs, a significantly higher obliteration rate was found with ruptured brain AVMs than unruptured brain AVMs, mainly accounted by SRS alone, with no protective advantage of embolization. While no significant differences were seen in the pooled findings, there was a tendency toward a decrease in the frequency of radiation-induced alterations and hemorrhagic rates following SRS alone, but with an increase in mortality. Therefore, SRS alone may confer a substantial benefit with a greater risk-to-reward ratio in achieving significant obliteration of nidus in patients presenting with ruptured brain AVMs, and therefore should be used without any adjunct embolization. Further investigation is necessary to fully understand the effects of pre-SRS embolization on both immediate and long-term consequences, including radiation-induced alterations, hemorrhaging, and mortality. These studies should consider confounding variables such as AVM angioarchitecture, patient variability, and the lack of standardization in techniques and regimens.
Consent
As this was a systematic review and meta-analysis, no consent was required.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
Abdul Basit Sangah has contributed the same amount of work as the first author.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abecassis IJ, Nerva JD, Feroze A, Barber J, Ghodke BV, Kim LJ. Multimodality management of spetzler-martin grade 3 brain arteriovenous malformations with subgroup analysis. World Neurosurg. 2017. 102: 263-74
2. Andrade-Souza YM, Ramani M, Scora D, Tsao MN, terBrugge K, Schwartz ML. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery. 2007. 60: 443-51 discussion 51-2
3. Antkowiak L, Putz M, Rogalska M, Mandera M. Multimodal treatment of pediatric ruptured brain arteriovenous malformations: A single-center study. Children (Basel). 2021. 8: 215
4. ApSimon HT, Reef H, Phadke RV, Popovic EA. A population-based study of brain arteriovenous malformation: Long-term treatment outcomes. Stroke. 2002. 33: 2794-800
5. Arai Y, Handa Y, Ishii H, Ueda Y, Uno H, Nakajima T. Endovascular therapy followed by stereotactic radiosurgery for cerebral arteriovenous malformations. Interv Neuroradiol. 2006. 12: 163-6
6. Back AG, Vollmer D, Zeck O, Shkedy C, Shedden PM. Retrospective analysis of unstaged and staged Gamma Knife surgery with and without preceding embolization for the treatment of arteriovenous malformations. J Neurosurg. 2008. 109: 57-64
7. Bethanabatla R, Spencer T, Kelly L, Gan P, Taha A. Stereotactic radio surgery, embolization and conservative management for cerebral arteriovenous malformation: A New Zealand experience of long-term outcomes. World Neurosurg. 2022. 164: e992-1000
8. Bokhari MR, Bokhari SR, editors. Arteriovenous malformation of the brain. StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. p.
9. Brown RD, Wiebers DO, Forbes G, O’Fallon WM, Piepgras DG, Marsh WR. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988. 68: 352-7
10. Brown RD, Wiebers DO, Torner JC, O’Fallon WM. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology. 1996. 46: 949-52
11. Bruno CA, Meyers PM. Endovascular management of arteriovenous malformations of the brain. Interv Neurol. 2013. 1: 109-23
12. Chang H, Silva MA, Weng J, Kovacevic J, Luther E, Starke RM. The impact of embolization on radiosurgery obliteration rates for brain arteriovenous malformations: A systematic review and meta-analysis. Neurosurg Rev. 2022. 46: 28
13. Chen CJ, Ding D, Lee CC, Kearns KN, Pomeraniec IJ, Cifarelli CP. Stereotactic radiosurgery with versus without embolization for brain arteriovenous malformations. Neurosurgery. 2021. 88: 313-21
14. Chen CJ, Lee CC, Ding D, Tzeng SW, Kearns KN, Kano H. Stereotactic radiosurgery for unruptured versus ruptured pediatric brain arteriovenous malformations. Stroke. 2019. 50: 2745-51
15. Chen JC, Mariscal L, Girvigian MR, Vanefsky MA, Glousman BN, Miller MJ. Hypofractionated stereotactic radiosurgery for treatment of cerebral arteriovenous malformations: Outcome analysis with use of the modified arteriovenous malformation scoring system. J Clin Neurosci. 2016. 29: 155-61
16. Chen Y, Li R, Ma L, Meng X, Yan D, Wang H. Long-term outcomes of brainstem arteriovenous malformations after different management modalities: A single-centre experience. Stroke Vasc Neurol. 2021. 6: 65-73
17. Crawford PM, West CR, Chadwick DW, Shaw MD. Arteriovenous malformations of the brain: Natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986. 49: 1-10
18. Dalyai R, eofanis T, Starke RM, Chalouhi N, Ghobrial G, Jabbour P. Stereotactic radiosurgery with neoadjuvant embolization of larger arteriovenous malformations: an institutional experience. Biomed Res Int. 2014. 2014: 306518
19. Darsaut TE, Guzman R, Marcellus ML, Edwards MS, Tian L, Do HM. Management of pediatric intracranial arteriovenous malformations: Experience with multimodality therapy. Neurosurgery. 2011. 69: 540-56 discussion 556
20. De Leacy R, Ansari SA, Schirmer CM, Cooke DL, Prestigiacomo CJ, Bulsara KR. Endovascular treatment in the multimodality management of brain arteriovenous malformations: Report of the Society of NeuroInterventional Surgery Standards and Guidelines Committee. J Neurointerv Surg. 2022. 14: 1118-24
21. Deruty R, Pelissou-Guyotat I, Morel C, Bascoulergue Y, Turjman F. Reflections on the management of cerebral arteriovenous malformations. Surg Neurol. 1998. 50: 245-55 discussion 55-6
22. Ding D, Starke RM, Sheehan JP. Radiosurgery for the management of cerebral arteriovenous malformations. Handb Clin Neurol. 2017. 143: 69-83
23. Ding D, Yen CP, Starke RM, Xu Z, Sun X, Sheehan JP. Radiosurgery for Spetzler-Martin Grade III arteriovenous malformations. J Neurosurg. 2014. 120: 959-69
24. Dumot C, Picart T, Eker O, Guyotat J, Berhouma M, Pelissou-Guyotat I. Outcomes of unruptured low-grade brain arteriovenous malformations using TOBAS (treatment of brain arteriovenous malformations study) criteria. World Neurosurg. 2022. 167: e1050-61
25. Eliava S, Gorozhanin V, Shekhtman O, Pilipenko Y, Kuchina O, Esposito G, Regli L, Cenzato M, Kaku Y, Tanaka M, Tsukahara T, editors. Surgical treatment of unruptured brain AVMs: Short-and long-term results. Trends in cerebrovascular surgery and interventions. Cham: Springer; 2021. p. 87-90
26. Elsenousi A, Aletich VA, Alaraj A. Neurological outcomes and cure rates of embolization of brain arteriovenous malformations with n-butyl cyanoacrylate or Onyx: A meta-analysis. J Neurointerv Surg. 2016. 8: 265-72
27. Erickson N, Mooney J, Salehani A, omas E, Ilyas A, Rahm S. Predictive factors for arteriovenous malformation obliteration after stereotactic radiosurgery: A single-center study. World Neurosurg. 2022. 160: e529-36
28. Faye M, Diallo M, Sghiouar M, Ndiaye Sy EC, Borius PY, Régis JM. Stereotactic radiosurgery for thalamus arteriovenous malformations. J Radiosurg SBRT. 2020. 6: 269-75
29. Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007. 356: 2704-12
30. Greenberg MS, editors. Handbook of neurosurgery. United States: Thieme; 2019. p.
31. Hasegawa T, Kato T, Naito T, Mizuno A, Koketsu Y, Hirayama K. Effect of embolization before stereotactic radiosurgery for brain arteriovenous malformations: A case-control study with propensity score matching. J Neurosurg. 2023. 138: 955-61
32. Haw CS, terBrugge K, Willinsky R, Tomlinson G. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006. 104: 226-32
33. Higgins Jomas JChandler JCumpston MLi TPage MJ. Cochrane handbook for systematic reviews of interventions. Available from: https://www.training.cochrane.org/handbook [Last accessed on 2024 Aug 23].
34. Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001. 95: 633-7
35. Hodgson TJ, Kemeny AA, Gholkar A, Deasy N. Embolization of residual fistula following stereotactic radiosurgery in cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 2009. 30: 109-10
36. Hoh BL, Ogilvy CS, Butler WE, Loeffler JS, Putman CM, Chapman PH. Multimodality treatment of nongalenic arteriovenous malformations in pediatric patients. Neurosurgery. 2000. 47: 346-57 discussion 57-8
37. Ilyas A, Chen CJ, Ding D, Buell TJ, Raper DMS, Lee CC. Radiation-induced changes after stereotactic radiosurgery for brain arteriovenous malformations: A systematic review and meta-analysis. Neurosurgery. 2018. 83: 365-76
38. Ilyas A, Chen CJ, Ding D, Taylor DG, Moosa S, Lee CC. Volume-staged versus dose-staged stereotactic radiosurgery outcomes for large brain arteriovenous malformations: A systematic review. J Neurosurg. 2018. 128: 154-64
39. Izawa M, Chernov M, Hayashi M, Iseki H, Hori T, Takakura K. Combined management of intracranial arteriovenous malformations with embolization and gamma knife radiosurgery: Comparative evaluation of the long-term results. Surg Neurol. 2009. 71: 43-52 discussion-3
40. Jiang X, Zhao Z, Zhang Y, Wang Y, Lai L. Preradiosurgery embolization in reducing the postoperative hemorrhage rate for patients with cerebral arteriovenous malformations: A systematic review and meta-analysis. Neurosurg Rev. 2021. 44: 3197-207
41. Jiang Z, Zhang X, Wan X, Wei M, Liu Y, Ding C. Efficacy and safety of combined endovascular embolization and stereotactic radiosurgery for patients with intracranial arteriovenous malformations: A systematic review and meta-analysis. Biomed Res Int. 2021. 2021: 6686167
42. Kano H, Kondziolka D, Flickinger JC, Park KJ, Iyer A, Yang HC. Stereotactic radiosurgery for arteriovenous malformations after embolization: A case-control study. J Neurosurg. 2012. 117: 265-75
43. Kato Y, Dong VH, Chaddad F, Takizawa K, Izumo T, Fukuda H. Expert consensus on the management of brain arteriovenous malformations. Asian J Neurosurg. 2019. 14: 1074-81
44. Kawashima M, Hasegawa H, Shin M, Shinya Y, Ishikawa O, Koizumi S. Outcomes of stereotactic radiosurgery for hemorrhagic arteriovenous malformations with or without prior resection or embolization. J Neurosurg. 2020. 135: 733-41
45. Kim BS, Yeon JY, Shin HS, Kim JS, Hong SC, Shin HJ. Gamma knife radiosurgery for incidental, symptomatic unruptured, and ruptured brain arteriovenous malformations. Cerebrovasc Dis. 2021. 50: 222-30
46. Kim EJ, Vermeulen S, Li FJ, Newell DW. A review of cerebral arteriovenous malformations and treatment with stereotactic radiosurgery. Transl Cancer Res. 2014. 3: 399-410
47. Kim MJ, Park SH, Park KY, Jung HH, Chang JH, Chang JW. Gamma knife radiosurgery followed by flow-reductive embolization for ruptured arteriovenous malformation. J Clin Med. 2020. 9: 1318
48. Kiran NA, Kale SS, Vaishya S, Kasliwal MK, Gupta A, Sharma MS. Gamma Knife surgery for intracranial arteriovenous malformations in children: A retrospective study in 103 patients. J Neurosurg. 2007. 107: 479-84
49. Laakso A, Hernesniemi J. Arteriovenous malformations: Epidemiology and clinical presentation. Neurosurg Clin N Am. 2012. 23: 1-6
50. Lecavalier-Barsoum M, Roy D, Doucet R, Fortin B, Lambert C, Moumdjian R. Long-term results of radiosurgery for cerebral arteriovenous malformations. Can J Neurol Sci. 2013. 40: 182-6
51. Lee CC, Chen CJ, Ball B, Schlesinger D, Xu Z, Yen CP. Stereotactic radiosurgery for arteriovenous malformations after Onyx embolization: A case-control study. J Neurosurg. 2015. 123: 126-35
52. Lenck S, Schwartz M, Hengwei J, Agid R, Nicholson P, Krings T. Management of residual brain arteriovenous malformations after stereotactic radiosurgery. World Neurosurg. 2018. 116: e1105-13
53. Li W, Wang Y, Lu L, Zhang Y. The factors associated with obliteration following stereotactic radiosurgery in patients with brain arteriovenous malformations: A meta-analysis. ANZ J Surg. 2022. 92: 970-9
54. Lindvall P, Grayson D, Bergström P, Bergenheim AT. Hypofractionated stereotactic radiotherapy in mediumsized to large arteriovenous malformations. J Clin Neurosci. 2015. 22: 955-8
55. Link TW, Winston G, Schwarz JT, Lin N, Patsalides A, Gobin P. Treatment of unruptured brain arteriovenous malformations: A single-center experience of 86 patients and a critique of the a randomized trial of unruptured brain arteriovenous malformations (ARUBA) trial. World Neurosurg. 2018. 120: e1156-62
56. Loebel F, Pontoriero A, Kluge A, Iatì G, Acker G, Kufeld M. Image-guided robotic radiosurgery for the treatment of arteriovenous malformations. PLoS One. 2022. 17: e0266744
57. Lv X, Song C, He H, Jiang C, Li Y. Transvenous retrograde AVM embolization: Indications, techniques, complications and outcomes. Interv Neuroradiol. 2017. 23: 504-9
58. Marciscano AE, Huang J, Tamargo RJ, Hu C, Khattab MH, Aggarwal S. Long-term outcomes with planned multistage reduced dose repeat stereotactic radiosurgery for treatment of inoperable high-grade arteriovenous malformations: An observational retrospective cohort study. Neurosurgery. 2017. 81: 136-46
59. Maruyama K, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brainstem arteriovenous malformations: Factors affecting outcome. J Neurosurg. 2004. 100: 407-13
60. Meng X, He H, Liu P, Gao D, Chen Y, Sun S. Radiosurgery-based AVM scale is proposed for combined embolization and gamma knife surgery for brain arteriovenous malformations. Front Neurol. 2021. 12: 647167
61. Milker-Zabel S, Kopp-Schneider A, Wiesbauer H, Schlegel W, Huber P, Debus J. Proposal for a new prognostic score for linac-based radiosurgery in cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2012. 83: 525-32
62. Mohr JP, Overbey JR, Hartmann A, Kummer RV, AlShahi Salman R, Kim H. Medical management with interventional therapy versus medical management alone for unruptured brain arteriovenous malformations (ARUBA): Final follow-up of a multicentre, non-blinded, randomised controlled trial. Lancet Neurol. 2020. 19: 573-81
63. Mooney J, Salehani A, Erickson N, omas E, Ilyas A, Rahm S. Stereotactic radiosurgery for ruptured versus unruptured intracranial arteriovenous malformations. Surg Neurol Int. 2022. 13: 194
64. Morel BC, Wittenberg B, Hoffman JE, Case DE, Folzenlogen Z, Roark C. Untangling the modern treatment paradigm for unruptured brain arteriovenous malformations. J Pers Med. 2022. 12: 904
65. Nagaraja S, Lee KJ, Coley SC, Capener D, Walton L, Kemeny AA. Stereotactic radiosurgery for brain arteriovenous malformations: quantitative MR assessment of nidal response at 1 year and angiographic factors predicting early obliteration. Neuroradiology. 2006. 48: 821-9
66. Nagy G, Grainger A, Hodgson TJ, Rowe JG, Coley SC, Kemeny AA. Staged-volume radiosurgery of large arteriovenous malformations improves outcome by reducing the rate of adverse radiation effects. Neurosurgery. 2017. 80: 180-92
67. Nagy G, Rowe JG, Radatz MW, Hodgson TJ, Coley SC, Kemeny AA. A historical analysis of single-stage. knife radiosurgical treatment for large arteriovenous malformations: evolution and outcomes. Acta Neurochir (Wien). 2012. 154: 383-94
68. Naoi Y, Iizuka Y, Cho N, Nakanishi A, Ito K, Akamatsu M. Stereotactic radiosurgery using a linear accelerator for the treatment of cerebral arteriovenous malformation: A preliminary report. J JASTRO. 2000. 12: 221-7
69. Nataraj A, Mohamed MB, Gholkar A, Vivar R, Watkins L, Aspoas R. Multimodality treatment of cerebral arteriovenous malformations. World Neurosurg. 2014. 82: 149-59
70. Nerva JD, Barber J, Levitt MR, Rockhill JK, Hallam DK, Ghodke BV. Onyx embolization prior to stereotactic radiosurgery for brain arteriovenous malformations: A single-center treatment algorithm. J Neurointerv Surg. 2018. 10: 258-67
71. Oermann EK, Ding D, Yen CP, Starke RM, Bederson JB, Kondziolka D. Effect of prior embolization on cerebral arteriovenous malformation radiosurgery outcomes: A case-control study. Neurosurgery. 2015. 77: 406-17
72. Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: A 24-year follow-up assessment. J Neurosurg. 1990. 73: 387-91
73. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
74. Paúl L, Casasco A, Kusak ME, Martínez N, Rey G, Martínez R. Results for a series of 697 arteriovenous malformations treated by gamma knife: Influence of angiographic features on the obliteration rate. Neurosurgery. 2014. 75: 568-83 dicussion 582-3; quiz 583
75. Peres CM, Souza EC, Teixeira MJ, Figueiredo EG, Caldas J. Impact of associated nidal lesions in outcome of brain arteriovenous malformations after radiosurgery with or without embolization. World Neurosurg. 2017. 105: 643-50
76. Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966. 25: 467-90
77. Plasencia AR, Santillan A. Embolization and radiosurgery for arteriovenous malformations. Surg Neurol Int. 2012. 3: S90-104
78. Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke. 1996. 27: 1-6
79. Pulli B, Chapman PH, Ogilvy CS, Patel AB, Stapleton CJ, Leslie-Mazwi TM. Multimodal cerebral arteriovenous malformation treatment: A 12-year experience and comparison of key outcomes to ARUBA. J Neurosurg. 2019. 133: 1792-801
80. Rajshekhar V, Moorthy RK, Jeyaseelan V, John S, Rangad F, Viswanathan PN. Results of a conservative dose plan linear accelerator-based stereotactic radiosurgery for pediatric intracranial arteriovenous malformations. World Neurosurg. 2016. 95: 425-33
81. Redekop GJ, Elisevich KV, Gaspar LE, Wiese KP, Drake CG. Conventional radiation therapy of intracranial arteriovenous malformations: Long-term results. J Neurosurg. 1993. 78: 413-22
82. Russell D, Peck T, Ding D, Chen CJ, Taylor DG, Starke RM. Stereotactic radiosurgery alone or combined with embolization for brain arteriovenous malformations: A systematic review and meta-analysis. J Neurosurg. 2018. 128: 1338-48
83. Rutledge C, Cooke DL, Hetts SW, Abla AA. Brain arteriovenous malformations. Handb Clin Neurol. 2021. 176: 171-8
84. Schlienger M, Atlan D, Lefkopoulos D, Merienne L, Touboul E, Missir O. Linac radiosurgery for cerebral arteriovenous malformations: Results in 169 patients. Int J Radiat Oncol Biol Phys. 2000. 46: 1135-42
85. Schwyzer L, Yen CP, Evans A, Zavoian S, Steiner L. Long-term results of gamma knife surgery for partially embolized arteriovenous malformations. Neurosurgery. 2012. 71: 1139-47 discussion 47-8
86. Stapf C, Mast H, Sciacca RR, Berenstein A, Nelson PK, Gobin YP. The New York Islands AVM Study: Design, study progress, and initial results. Stroke. 2003. 34: e29-33
87. Statham P, Macpherson P, Johnston R, Forster DM, Adams JH, Todd NV. Cerebral radiation necrosis complicating stereotactic radiosurgery for arteriovenous malformation. J Neurol Neurosurg Psychiatry. 1990. 53: 476-9
88. Sun DQ, Carson KA, Raza SM, Batra S, Kleinberg LR, Lim M. The radiosurgical treatment of arteriovenous malformations: Obliteration, morbidities, and performance status. Int J Radiat Oncol Biol Phys. 2011. 80: 354-61
89. Talaat M, Premat K, Lenck S, Shotar E, Boch AL, Bessar A. Exclusion treatment of ruptured and unruptured low-grade brain arteriovenous malformations: A systematic review. Neuroradiology. 2022. 64: 5-14
90. Tao JJ, Moore J, Appelboom G, Chang SD, Trifiletti DM, Chao ST, Sahgal A, Sheehan JP, editors. Stereotactic radiosurgery for intracranial arteriovenous malformations. Stereotactic radiosurgery and stereotactic body radiation therapy. A comprehensive guide. Cham: Springer International Publishing; 2019. p. 131-40
91. Thenier-Villa JL, Galárraga-Campoverde RA, Martínez Rolán RM, De La Lama Zaragoza AR, Martínez Cueto P, Muñoz Garzón V. Linear accelerator stereotactic radiosurgery of central nervous system arteriovenous malformations: A 15-year analysis of outcome-related factors in a single tertiary center. World Neurosurg. 2017. 103: 291-302
92. Tokarev A, Rak V, Terekhin I, Neznanova M, Evdokimova O, Stepanov V. Complications after stereotactic radiosurgery in patients with brain disorders. Russ J Neurosurg. 2022. 23: 18-32
93. Tonetti DA, Gross BA. Re-evaluating clinical outcomes for AVM stereotactic radiosurgery. Prog Neurol Surg. 2019. 34: 267-72
94. van Beijnum J, van der Worp HB, Buis DR, Al-Shahi Salman R, Kappelle LJ, Rinkel GJ. Treatment of brain arteriovenous malformations: A systematic review and meta-analysis. JAMA. 2011. 306: 2011-9
95. Vollherbst DF, Chapot R, Bendszus M, Möhlenbruch MA. Glue, onyx, squid or PHIL? liquid embolic agents for the embolization of cerebral arteriovenous malformations and dural arteriovenous fistulas. Clin Neuroradiol. 2022. 32: 25-38
96. Waqas M, Monteiro A, Tutino VM, Cappuzzo JM, Vakharia K, Winograd EK. Preradiosurgical embolization of arteriovenous malformations reduces target volume-the main determinant for complete obliteration. World Neurosurg. 2024. 181: e117-25
97. Weber W, Kis B, Siekmann R, Jans P, Laumer R, Kühne D. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007. 61: 244-52 discussion 52-4
98. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. UK: University of Liverpool; 2000. p.
99. Winkler EA, Lu A, Morshed RA, Yue JK, Rutledge WC, Burkhardt JK. Bringing high-grade arteriovenous malformations under control: Clinical outcomes following multimodality treatment in children. J Neurosurg Pediatr. 2020. 26: 82-91
100. Xiaochuan H, Yuhua J, Xianli L, Hongchao Y, Yang Z, Youxiang L. Targeted embolization reduces hemorrhage complications in partially embolized cerebral AVM combined with gamma knife surgery. Interv Neuroradiol. 2015. 21: 80-7
101. Xu F, Zhong J, Ray A, Manjila S, Bambakidis NC. Stereotactic radiosurgery with and without embolization for intracranial arteriovenous malformations: A systematic review and meta-analysis. Neurosurg Focus. 2014. 37: E16
102. Yan D, Chen Y, Li Z, Zhang H, Li R, Yuan K. Stereotactic radiosurgery with vs. without prior embolization for brain arteriovenous malformations: A propensity score matching analysis. Front Neurol. 2021. 12: 752164
103. Yang SY, Kim DG, Chung HT, Paek SH, Park JH, Han DH. Radiosurgery for large cerebral arteriovenous malformations. Acta Neurochir (Wien). 2009. 151: 113-24
104. Zhu D, Li Z, Zhang Y, Fang Y, Li Q, Zhao R. Gamma knife surgery with and without embolization for cerebral arteriovenous malformations: A systematic review and meta-analysis. J Clin Neurosci. 2018. 56: 67-73
105. Zhu S, Brodin P, Garg M, LaSala P, Tome W. Systematic review and meta-analysis of the dose-response and risk factors for obliteration of arteriovenous malformations following radiosurgery: An update based on the last 20 years of published clinical evidence. Neurosurg Open. 2021. 2: okab004