- Department of Neurosurgery and Surgical Specialties, Pedro Ernesto University Hospital, Universidade do Estado do Rio de Janeiro, Brazil
- Department of Pathology, Pedro Ernesto University Hospital, Universidade do Estado do Rio de Janeiro, Brazil
- Department of Neurosurgery, State Institute of Brain Paulo Niemeyer, Rio de Janeiro, Brazil.
Correspondence Address:
João Antonio Gonçalves Bastos Torres, Department of Neurosurgery and Surgical Specialties, Pedro Ernesto University Hospital, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil.
DOI:10.25259/SNI_34_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: João Antonio Gonçalves Bastos Torres1, Pedro Henrique Costa Ferreira-Pinto1, Domênica Baroni Coelho de Oliveira Ferreira1, Elington Lannes Simões1, Felipe Gonçalves de Carvalho1, Ana Carolina Gonçalves Brito2, José Alberto Almeida Filho3, Bruna Cavalcante de Sousa1, Maria Eduarda Viveiros de Castro1, Pedro Luiz Ribeiro Carvalho de Gouvea1, Wellerson Novaes da Silva1, Eduardo Mendes Correa1, Thainá Zanon Cruz1, Flavio Nigri1. Endolymphatic sac tumor: An urgent case presenting acute intracranial hypertension successfully treated with suboccipital decompressive craniectomy – 8 years of follow-up. 17-May-2024;15:166
How to cite this URL: João Antonio Gonçalves Bastos Torres1, Pedro Henrique Costa Ferreira-Pinto1, Domênica Baroni Coelho de Oliveira Ferreira1, Elington Lannes Simões1, Felipe Gonçalves de Carvalho1, Ana Carolina Gonçalves Brito2, José Alberto Almeida Filho3, Bruna Cavalcante de Sousa1, Maria Eduarda Viveiros de Castro1, Pedro Luiz Ribeiro Carvalho de Gouvea1, Wellerson Novaes da Silva1, Eduardo Mendes Correa1, Thainá Zanon Cruz1, Flavio Nigri1. Endolymphatic sac tumor: An urgent case presenting acute intracranial hypertension successfully treated with suboccipital decompressive craniectomy – 8 years of follow-up. 17-May-2024;15:166. Available from: https://surgicalneurologyint.com/surgicalint-articles/12896/
Abstract
Background: Endolymphatic sac tumor (ELST) is a rare lesion. It may be sporadically or associated with Von Hippel-Lindau syndrome. Progressive audiovestibular symptoms characterize the typical clinical presentation. Here, we report a unique case of ELST with acute intracranial hypertension (IH) due to tumor compression, successfully treated with an urgent suboccipital decompressive craniectomy (SDC).
Case Description: A 33-year-old woman previously underwent a biopsy and ventriculoperitoneal shunt. The histopathological finding revealed an ELST. One year later, she developed headache, vomiting, and somnolence due to brainstem compression. An urgent SDC was performed. One month later, preoperative endovascular embolization and partial tumor resection were carried out. After 6 months adjuvant radiotherapy (RT) therapy was administered. She has been under follow-up for 8 years since the last surgical procedure, and the tumor remains stable.
Conclusion: ELST generally has a progressive clinical course. This is a unique case with acute IH due to tumor compression. The tumor’s high vascularity and the unavailability of endovascular embolization precluded its resection. SDC was an alternative approach. The final treatment included tumor embolization, surgical resection, and RT. No progression was observed for 8 years after the last procedure, and long-term follow-up is warranted.
Keywords: Adjuvant radiotherapy, Decompressive suboccipital craniectomy, Endolymphatic sac tumor, Intracranial hypertension, Tumor embolization
INTRODUCTION
Endolymphatic sac tumor (ELST) is a rare, locally invasive, low-grade adenocarcinoma that arises from the endolymphatic epithelium within the vestibular aqueduct.[
CLINICAL PRESENTATION
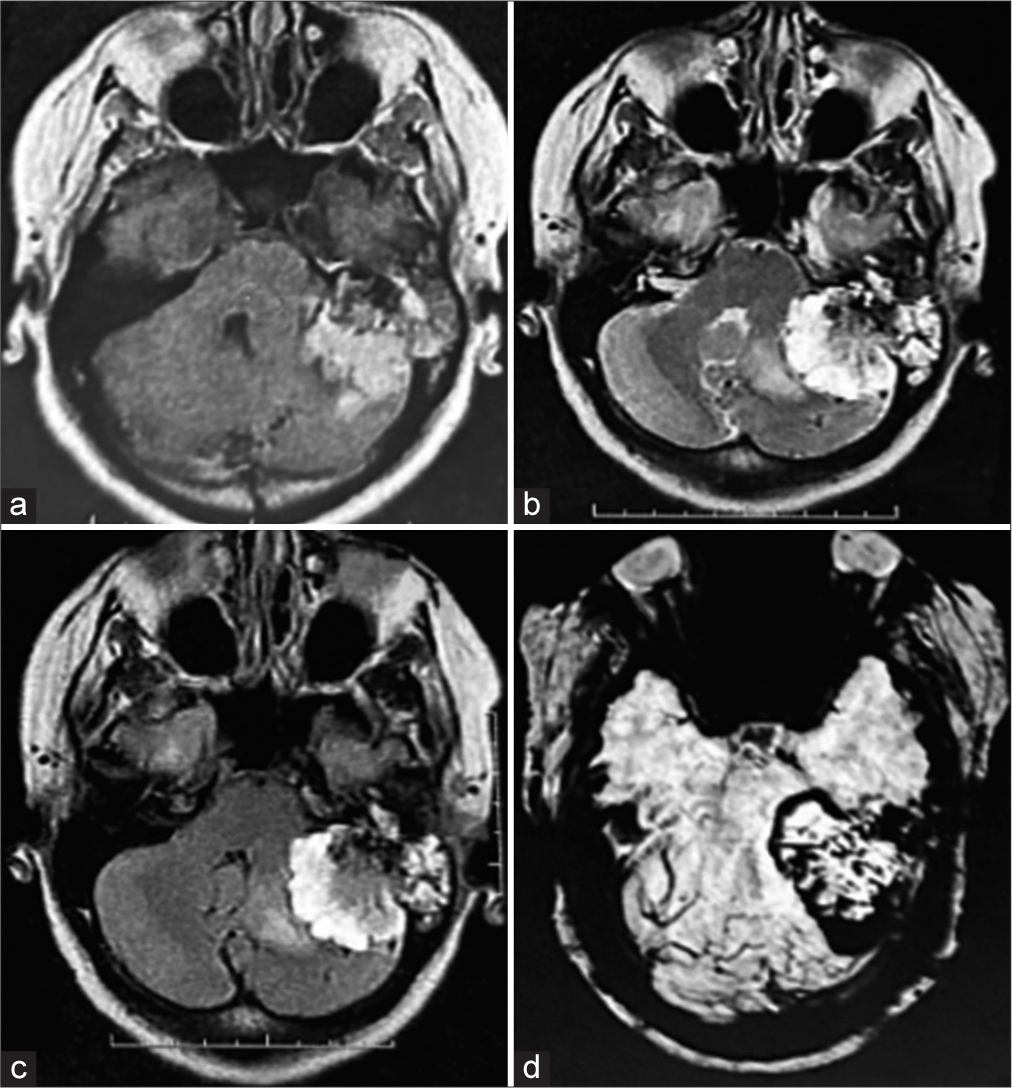
A 33-year-old female patient was admitted to the university hospital with a headache, loss of balance, and progressive left-sided hearing loss. On physical examination, she presented left-sided deafness and left-sided facial paralysis: House–Brackmann grade 3. Other cranial nerves were normal. Head computed tomography (CT) findings revealed a bulky enhancement soft-tissue mass (maximum transverse diameter of 4.2 cm × 3.6 cm) of the left posterior cranial fossa and temporo-occipital region that had eroded the base bone from the skull to the middle and posterior cranial fossa [
Figure 2:
Preoperative brain magnetic resonance imaging showing a lesion located in the left temporal: Petrous and mastoid part invading the left cerebellopontine angle. (a) T1 and (b) T2 weighted. (c) Fluid attenuated inversion recovery image and (d) T2 gradient echo exhibiting peripheral hypointensity, suggesting a high vascularization pattern.
The translabyrinthine approach, including a traditional curvilinear retroauricular incision, was the first surgical procedure. The Neurosurgery and Head and Neck Surgery teams worked together. After the dural opening, there was intense bleeding from the large mass, and the procedure had to be interrupted. The right parietal ventriculoperitoneal shunt was also performed without any complications.
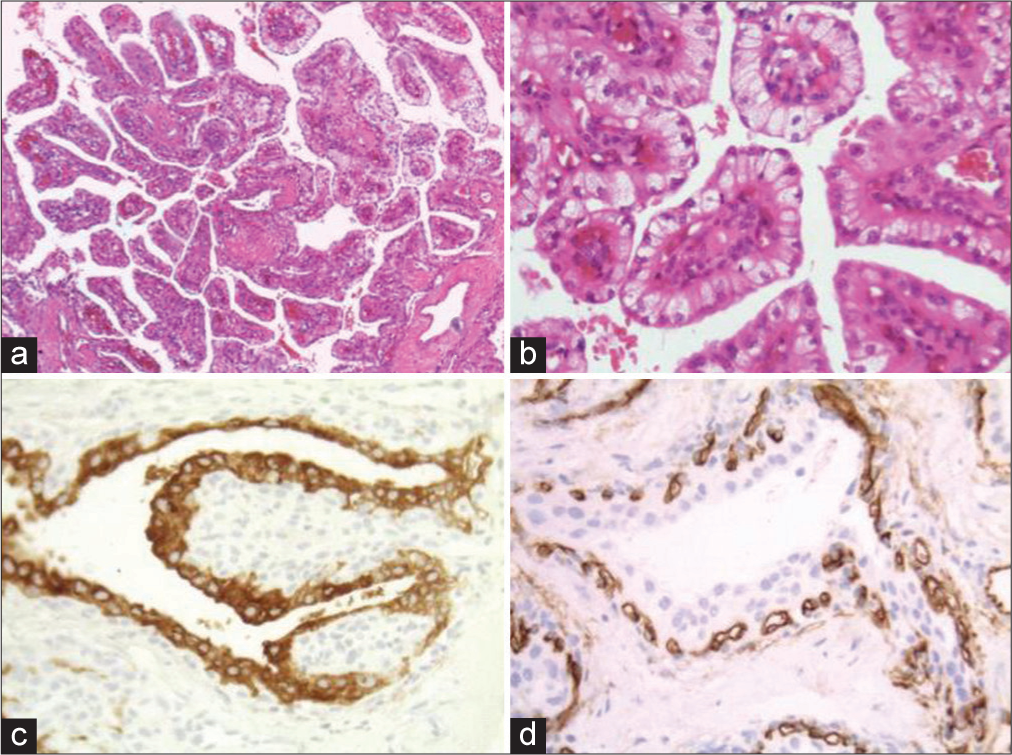
Samples from the temporal left bone histologically revealed a neoplasm with a papillary pattern with a dense fibrous core presenting proliferated and congested capillaries and cuboidal to the columnar epithelial lining with clear or eosinophilic cytoplasm [
Figure 4:
Samples from the temporal bone region: (a) Hematoxylin and Eosin (H&E) stain (×200). Neoplasm of a florid papillary arrangement with a dense fibrous core presenting proliferated and congested capillaries and cuboidal to columnar epithelial lining. (b) H&E stain (×400). Epithelial cells have clear or eosinophilic cytoplasm, recovering the papillary structures in a monolayer. (c) Epithelial membrane antigen (EMA) (×400). The cuboidal to columnar epithelial recovering the papillae staining positively for EMA. (d) CD34 (×400). Capillaries are highlighted by CD34 immunopositivity.
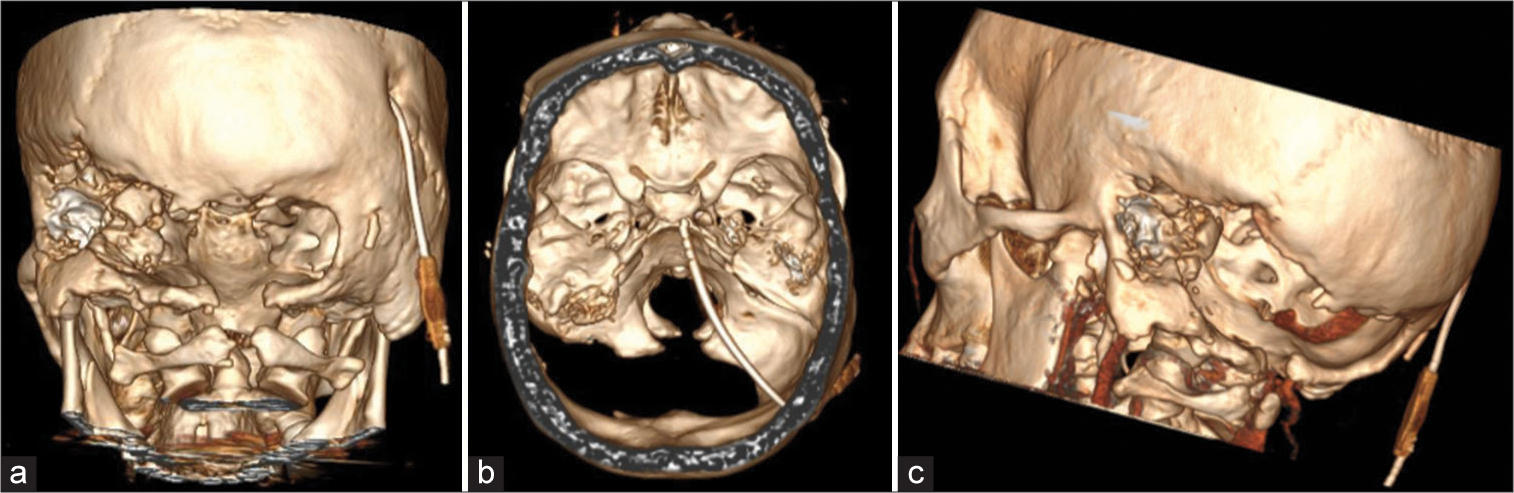
One year after the tumor biopsy and virtual pathology slide (VPS), the patient was readmitted with headaches and vomiting. One week after admission, the patient presented with somnolence and subsequently loss of consciousness due to direct compression of the brainstem by the tumor and herniation of the cerebellar tonsils. There was no hydrocephalus and the VPS functioning was normal. An emergency SDC was performed. The procedure included a midline incision followed by an autologous pericranium dural augmentation [
Figure 5:
Head CT 3D image reconstruction using RadiAnt DICOM Viewer Software (Medixant, Poznan, Poland). (a) Posterior view exhibiting the posterior fossa decompression with left extension. It is possible to observe the virtual pathology slide burr hole on the right side. (b) Superior view. (c) Oblique view.
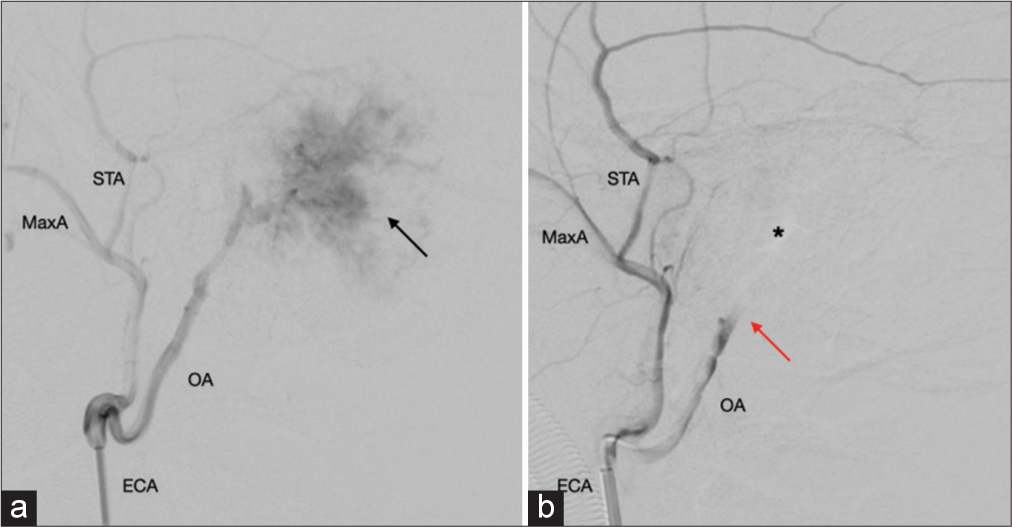
One month after the SDC, endovascular embolization of the tumor was performed at another institution. The angiogram revealed that the tumor’s blood supply was from the external carotid artery. The occipital artery branch embolization was substantially effective in reducing tumor vascularization. The angiogram images were processed using the RadiAnt DICOM Viewer Software (Medixant, Poznan, Poland) [
Figure 6:
Head and neck angiography. (a) The angiogram revealed a high vascularization area from branches of the occipital artery compatible with the tumor feeding (black arrow). (b) After tumor endovascular embolization, there was a considerable blood flow interruption (red arrow). Asterix represents the tumor area. ECA: External carotid artery, MaxA: Maxillary artery, STA: Superficial temporal artery, OA: Occipital artery.
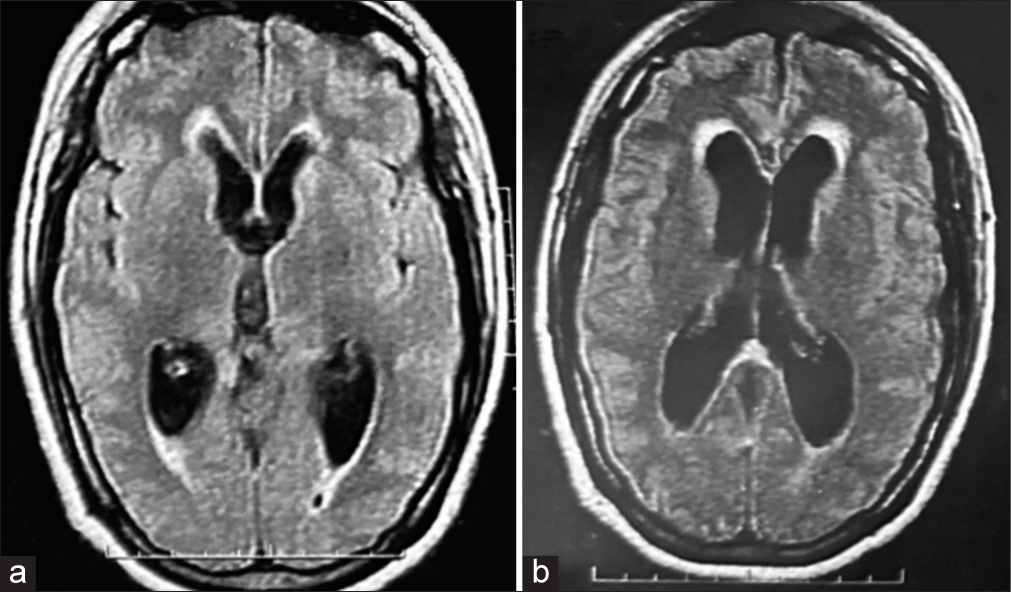
Figure 7:
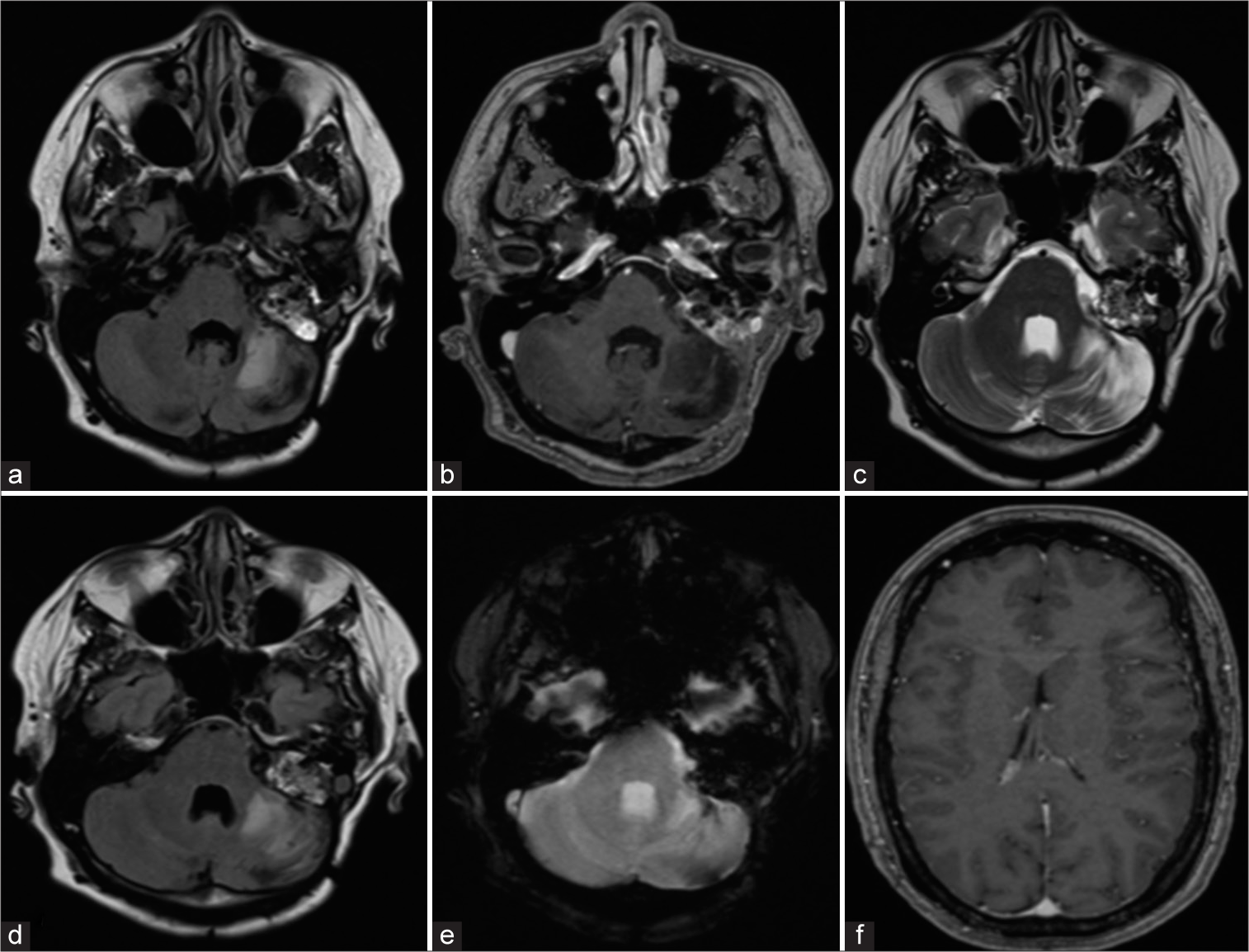
Follow-up brain magnetic resonance imaging in (a) T1 weighted, (b) T1 contrast enhancement, (c) T2 weighted, (d) fluid-attenuated inversion recovery image, (e) T2 gradient echo, and (f) axial T1-weighted. (a-e) The residual tumor remains unchanged after 8 years of the last surgical procedure. (c-d) Images show cerebellar parenchymal hyperintensity due to late postradiation effects. (f) Ventricles without hydrocephalus are observed.
DISCUSSION
ELSTs are characterized as low-grade invasive adenocarcinomas, affecting the petrous and mastoid portion of the temporal bone. The tumor arises from the vestibular aqueduct of the endolymphatic sac. Big tumors can also invade the cerebellopontine angle.[
The common clinical presentation includes hearing loss, tinnitus, vertigo, aural fullness, and facial nerve dysfunction.[
The most common treatment options are observation (in exceptional cases), surgical resection, RT, and endovascular embolization.[
Preoperative tumor embolization may be reasonable for huge and highly vascularized tumors. According to Ge et al.,[
The efficacy and indication of adjuvant RT are not well defined. Some authors advise RT for positive margins or subtotal resection.[
Considering the inconsistency and incomplete data regarding the extent of surgical resection, it is difficult to measure the impact of adjuvant therapy, such as RT and preoperative embolization. Moreover, data about the outcomes, including progression and recurrence, are not well defined.[
Wait-and-see strategy is not widely described in the literature. Tang et al.[
In our case, we attempted observation after the first approach (biopsy and VPS), considering that the tumor was a low-grade neoplasm, locally invasive, and with slow clinical progression. Wait-and-see was unsuccessful as after a year, the patient was worse with acute IH. Due to the severity of her condition and the unavailability of embolization, a decompressive craniectomy was performed. Although patients usually underwent this procedure in traumas,[
After clinical stabilization and availability of embolization, we chose endovascular preoperative embolization and tumor resection based on the assessment of age and good prognosis. It was not necessary to have a cranioplasty performed. Due to a remnant of a tumor inside the petrous and mastoid part of the temporal bone, we decided to adjuvant RT. After 10 years of the diagnosis and 8 years after the last surgery, the tumor remains stable, and the patient is clinically well. This demonstrates that surgical treatment combined with RT and embolization can lead to a better prognosis.
CONCLUSION
Although ELST generally has a slowly progressive clinical course, this unique case presented with acute IH due to tumor compression. The tumor’s high vascularity and the unavailability of endovascular embolization made its resection unfeasible. To deal with this emergency, SDC was performed. After clinical stabilization, the patient underwent preoperative tumor embolization and partial resection. No tumor progression was observed during the 8 years after the last surgical procedure. Long-term follow-up is warranted.
Ethical approval
Institutional review board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Rio de Janeiro State Research Support Foundation – Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Center of High Complexity Neurosurgery Intern Patients – Núcleo de Internação de Pacientes Neurocirúrgicos de Alta Complexidade (NIPNAC) – Rio de Janeiro State Health Secretary.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bausch B, Wellner U, Peyre M, Boedeker CC, Hes FJ, Anglani M. Characterization of endolymphatic sac tumors and von Hippel-Lindau disease in the International Endolymphatic Sac Tumor Registry. Head Neck. 2016. 38: E673-9
2. Bertani R, Koester SW, Perret C, Pilon B, Batista S, Brocco B. Decompressive hemicraniectomies as a damage control approach for multilobar firearm projectile injuries: A single-center experience. World Neurosurg. 2023. 169: e96-101
3. Elktaibi A, Damiri A, Rharrassi I, Elochi MR, Oukabli M, Akhaddar A. A rare tumour in the cerebellopontine angle: Endolymphatic sac tumour. Pan Afr Med J. 2018. 31: 127
4. Ge H, Wang H, Cai J, Zhang X, Mei W, Wu X. Endolymphatic sac tumor: Case report and literature review. Chin Neurosurg J. 2020. 6: 16
5. Guo F, Zhang L, Mo L. Long experience for the diagnosis and treatment of sporadic endolymphatic sac tumor in a single center. Clin Neurol Neurosurg. 2020. 197: 106078
6. Hassard AD, Boudreau SF, Cron CC. Adenoma of the endolymphatic sac. J Otolaryngol. 1984. 13: 213-6
7. Kim HJ, Hagan M, Butman JA, Baggenstos M, Brewer C, Zalewski C. Surgical resection of endolymphatic sac tumors in von Hippel-Lindau disease: Findings, results, and indications. Laryngoscope. 2013. 123: 477-83
8. Li JC, Brackmann DE, House JW, Lo WW, Carberry JN. Reclassification of aggressive adenomatous mastoid neoplasms as endolymphatic sac tumors. Laryngoscope. 1993. 103: 1342-8
9. Lonser RR, Baggenstos M, Kim HJ, Butman JA, Vortmeyer AO. The vestibular aqueduct: site of origin of endolymphatic sac tumors. J Neurosurg. 2008. 108: 751-6
10. Maher ER, Kaelin WG. von Hippel-Lindau disease. Medicine (Baltimore). 1997. 76: 381-91
11. Manski TJ, Heffner DK, Glenn GM, Patronas NJ, Pikus AT, Katz D. Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA. 1997. 277: 1461-6
12. Medixa. RadiAnt DICOM viewer. Version 2021. 2021. p. Available from: https://www.radiantviewer.com [Last accessed on 2024 Jan 08]
13. Mendenhall WM, Suárez C, Skálová A, Strojan P, Triantafyllou A, Devaney KO. Current treatment of endolymphatic sac tumor of the temporal bone. Adv Ther. 2018. 35: 887-98
14. Nelson T, Hu J, Bannykh S, Fan X, Rudnick J, Vail E. Clinical response to pazopanib in a patient with endolymphatic sac tumor not associated with von Hippel-Lindau syndrome. CNS Oncol. 2020. 9: CNS50
15. Patankar AP, Aiyer R, Chamarajan S, Vala N. Endolymphatic sac tumour: A case report and review of the literature. Arq Bras Neurocir. 2021. 40: e387-93
16. Patel NP, Wiggins RH, Shelton C. The radiologic diagnosis of endolymphatic sac tumors. Laryngoscope. 2006. 116: 40-6
17. Poulsen ML, Gimsing S, Kosteljanetz M, Møller HU, Brandt CA, Thomsen C. von Hippel-Lindau disease: Surveillance strategy for endolymphatic sac tumors. Genet Med. 2011. 13: 1032-41
18. Schipper J, Maier W, Rosahl S, Berlis A, Laszig R. Stadiengerechte Chirurgie von Saccus-endolymphaticusTumoren (ELST) [Tumour staged surgery of endolymphatic sac tumors (ELST)]. Laryngorhinootologie. 2004. 83: 493-500
19. Tang JD, Grady AJ, Nickel CJ, Ryan LE, Malone A, Canvasser L. Systematic review of endolymphatic sac tumor treatment and outcomes. Otolaryngol Head Neck Surg. 2023. 168: 282-90
20. Zanoletti E, Girasoli L, Borsetto D, Opocher G, Mazzoni A, Martini A. Endolymphatic sac tumour in von Hippel-Lindau disease: Management strategies. Acta Otorhinolaryngol Ital. 2017. 37: 423-9