- Interventional Neuroradiology, Hospital Israelita Albert Einstein, São Paulo, Brazil
- Department of Biomedical Engineering (2016-2017), Arizona State University, Tempe, United States
- Neurosurgery Division, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, United States
- Department of Radiology, University of Pittsburgh Medical Center, Pittsburgh, United States
- Department of Radiology, NYU Langone Health, New York, United States
- Boulder Statistics LLC, Boulder, United States
Correspondence Address:
Rafael Trindade Tatit, Interventional Neuroradiology, Hospital Israelita Albert Einstein, São Paulo, Brazil. .
DOI:10.25259/SNI_1118_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rafael Trindade Tatit1, Carlos Eduardo Baccin1, Priya Nair2, Emmanuel O. Mensah3, James Ryan Mason4, Seena Dehkharghani5, Karen Copeland6, Christopher S. Ogilvy3. Enhancing precision in aneurysm volume measurement: A comparative study of techniques including an artificial intelligence-based method for endovascular coiling. 30-May-2025;16:213
How to cite this URL: Rafael Trindade Tatit1, Carlos Eduardo Baccin1, Priya Nair2, Emmanuel O. Mensah3, James Ryan Mason4, Seena Dehkharghani5, Karen Copeland6, Christopher S. Ogilvy3. Enhancing precision in aneurysm volume measurement: A comparative study of techniques including an artificial intelligence-based method for endovascular coiling. 30-May-2025;16:213. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13590
Abstract
Background: Durable occlusion after endovascular coiling can be compromised by recanalization, underscoring the need for accurate cerebral aneurysm assessment. Precise volume measurement not only informs treatment decisions and detects subtle aneurysm growth but also refines calculations of packing density, historically linked to occlusion success. This study compares three volume-measurement approaches-traditional two-dimensional (2D) estimation, a semi-automated three-dimensional (3D) technique, and an artificial intelligence (AI)-based 3D method.
Methods: In this retrospective analysis, 24 aneurysms were assessed using 3D rotational angiography. Manual segmentation by three specialists using ITK-SNAP or mimics served as the reference standard. These results were compared with volumes from a semi-automated 3D platform (Philips Advanced Visualization Workspace), an AI-based tool (RapidAI for Aneurysm), and traditional 2D estimations. Agreement with the reference standard was quantified through Passing-Bablok regression slopes and mean biases.
Results: Passing-Bablok slopes for the 2D, Philips, and RapidAI methods were 0.83, 0.87, and 0.94, respectively, while mean biases were –24.7 mm3 (2D), –19.5 mm3 (Philips), and –14.5 mm3 (RapidAI). RapidAI demonstrated the strongest correlation with the reference standard, whereas 2D estimations showed the largest discrepancy. The semi-automated 3D method exhibited intermediate accuracy, potentially influenced by the clinician input required for segmentation.
Conclusion: All methods underestimated aneurysm volumes compared to the reference standard, suggesting that inaccurate volume measurements may mask early aneurysm growth. Among the techniques assessed, the AI-based approach provided the closest agreement with the reference, indicating that improved volumetric methods-particularly AI-driven ones-can enhance early detection of aneurysm expansion, guide treatment decisions, and help establish more reliable follow-up strategies for both treated and conservatively managed aneurysms.
Keywords: Artificial intelligence, Endovascular coiling, Packing density, Volumetric analysis
INTRODUCTION
Background
Endovascular coiling is widely used to treat cerebral aneurysms and help prevent rupture or re-rupture events.[
While packing density has historically been associated with reduced recanalization rates,[
Conventionally, aneurysm volume estimation relies on 2D measurements derived from angiographic images. Although convenient, these methods often oversimplify the complex three-dimensional (3D) shape of aneurysms, resulting in inaccurate volume estimation and, consequently, imprecise calculations of packing density.[
Objectives
This study compares traditional 2D estimations, a semiautomated 3D method, and an AI-based 3D platform against a reference standard established by manual segmentation performed by three experienced specialists using ITK-SNAP or Mimics. By evaluating each technique’s bias and statistical agreement with the reference standard, we aim to determine the most precise and consistent method for aneurysm volume measurement. We also discuss the broader clinical implications of accurate volumetric assessments for patients undergoing endovascular coiling or long-term follow-up, underscoring how refined measurement tools can guide critical treatment decisions and potentially reduce adverse events.
MATERIALS AND METHODS
Study design and patient selection
A retrospective analysis was conducted on 24 cerebral aneurysms from patients who underwent pre-interventional 3D rotational angiography (3DRA) at our institution between February 2015 and August 2020. The Institutional Review Board approval was obtained from the Research Ethics Committee of the Hospital Israelita Albert Einstein and the National Research Ethics Commission (CAAE: 39713520.0.0000.0071; Approval Number: 4.897.322). Informed consent was obtained from all participants before inclusion in the study.
Reference standard volume measurements
The reference standard for aneurysm volume measurement was established through manual segmentation performed independently by three experienced specialists using ITK-SNAP or Mimics software-advanced tools for medical image segmentation that facilitate precise 3D delineation of anatomical structures. Pre-interventional 3DRA images were acquired using standard imaging protocols to ensure high resolution and optimal image quality for detailed analysis. The Digital Imaging and Communications in Medicine image sets were then imported into the segmentation software.
Each specialist independently performed manual segmentation by meticulously outlining the aneurysm boundaries on individual slices of the 3DRA image stack. Utilizing the software’s manual tracing tools, they carefully delineated the aneurysm walls, accounting for complex morphologies, including irregular shapes and lobulations. Following the slice-by-slice segmentation, the software generated a 3D model of the aneurysm, providing a visual representation that allowed for verification and refinement of segmentation accuracy. The software then calculated the aneurysm volume by summing the volumes of all voxels enclosed within the segmented boundaries, with voxel dimensions derived from the imaging parameters of the 3DRA acquisition.
To minimize bias, each specialist was blinded to the measurements obtained by the others. The reference standard volume for each aneurysm was determined by averaging the individual volume measurements from the three specialists, thereby reducing interobserver variability and enhancing the reliability of the reference standard.
Comparison methods
We compared aneurysm volume measurements obtained from three different methods against the reference standard established by manual segmentation from specialists.
Traditional 2D linear estimation
Aneurysm volumes were estimated using 2D measurements performed by a radiologist. Maximum aneurysm diameters in three orthogonal planes (D1, D2, and D3) were obtained from 2D digital subtraction angiography images. Depending on the aneurysm’s shape, volumes were calculated using AngioCalc (www.angiocalc.com), a cerebral aneurysm volume and packing density analysis software. The formulas employed were as follows:
• Ellipsoid formula (used for ellipsoidal aneurysms):
Where D1, D2, and D3 are the maximum diameters of the aneurysm in three orthogonal planes.
• Sphere formula (used for spherical aneurysms):
Where D is the maximum diameter of the aneurysm.
All measurements and calculations were performed in AngioCalc, which is designed to standardize volume estimation and packing density analysis. This method provides a simplified estimation based on the aneurysm’s assumed geometric shape.
Semi-automated 3D technique (Philips advanced visualization workspace)
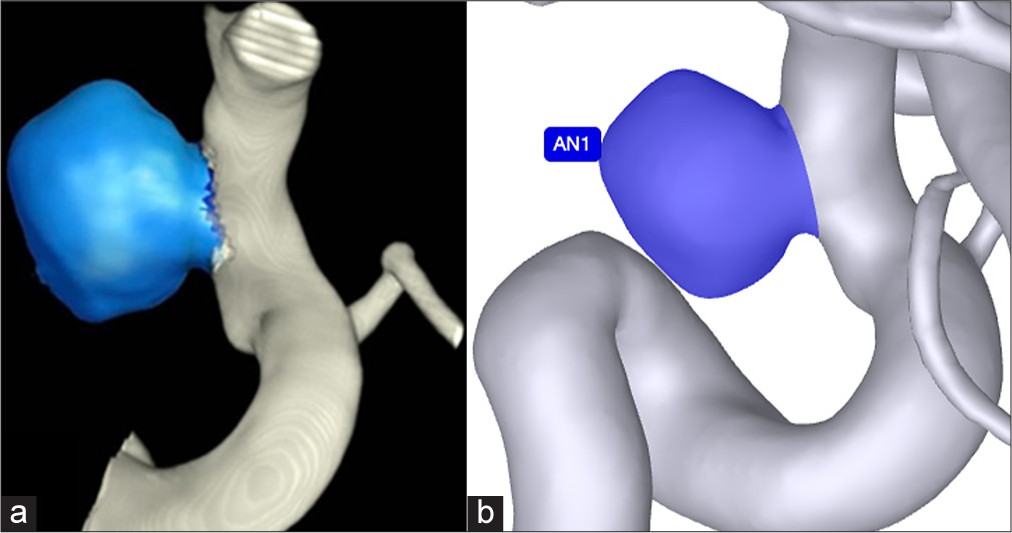
The Philips Advanced Visualization Workspace (Koninklijke Philips NV) software provides a semi-automated approach for aneurysm volume measurement using 3DRA images. This process involves several steps, beginning with the clinician selecting the aneurysm’s region of interest (ROI) on the 3DRA images. Once the ROI is defined, the clinician adjusts segmentation parameters, such as threshold values, to outline the aneurysm boundaries accurately. Based on these boundaries, the software generates a 3D model of the aneurysm [
AI-based 3D method (RapidAI for aneurysm)
RapidAI for Aneurysm (iSchema View Inc.) is an artificial intelligence (AI)-based software that automates segmentation and volume calculation for aneurysms using 3DRA images. The workflow begins with the software automatically detecting the aneurysm by applying machine learning algorithms trained on aneurysm imaging data [
Data processing and validation
For each aneurysm, volume measurements obtained from the three described methods were compared to the reference standard volumes derived from the averaged manual segmentations by the three specialists. These comparisons were conducted to assess the accuracy, bias, and variability of each method relative to the reference standard.
Statistical analysis
Statistical analyses were performed using JMP (SAS Institute, Cary, NC, USA). The agreement between each volume measurement method and the reference standard was evaluated using Passing-Bablok regression analysis to assess proportional and systematic differences between methods. Bland-Altman plots were generated to visualize mean biases and limits of agreement between each method and the reference standard. The mean bias was calculated as the average difference between the method’s volume measurements and the reference standard volumes. P < 0.05 was considered statistically significant for all analyses.
RESULTS
Patient and aneurysm characteristics
A total of 24 cerebral aneurysms were analyzed, with the majority located in the internal carotid artery (n = 10), followed by the middle cerebral artery (n = 7), the anterior cerebral artery (n = 4), and the basilar artery tip (n = 1). The aneurysm volumes measured by the reference standard method-manual segmentation by specialists-ranged from 10.56 mm3 to 344.71 mm3, with a mean ± standard deviation of 109.39 mm3 ± 101.90 mm3.
Comparison of volume measurement methods
The aneurysm volumes obtained using the three different measurement methods-traditional 2D linear estimation, semi-automated 3D measurement using the Philips Advanced Visualization Workspace, and AI-based 3D measurement using RapidAI for Aneurysm-were compared to the reference standard volumes.
Passing-Bablok regression analysis
Passing-Bablok regression analyses were performed to assess the agreement between each volume measurement method and the reference standard [
Bland-Altman analysis
Bland-Altman plots were generated to visualize the mean biases and limits of agreement between each measurement method and the reference standard [
Figure 2:
Bland-Altman plots illustrating the agreement between the reference standard and each aneurysm volume measurement method: (a) Traditional two-dimensional linear estimation, (b) semi-automated three-dimensional volumetric technique (Philips Advanced Visualization Workspace), and (c) artificial intelligence-based volumetric method (RapidAI for Aneurysm). Top panels: Scatter plots with Passing-Bablok regression line (red) and identity line y = x (blue dotted line), illustrating the relationship between each method and the reference standard. Bottom panels: Bland-Altman plots with solid red lines indicating the mean bias and dashed red lines representing the 95% limits of agreement. P-values in orange with asterisks (*) denote statistically significant biases (paired t-test).
The negative mean biases observed across all methods indicate that each underestimated aneurysm volumes compared to the reference standard. Among the evaluated methods, the RapidAI for Aneurysm demonstrated the least underestimation, as reflected by its smaller mean bias.
Summary of findings
Collectively, these results indicate that the AI-based RapidAI for Aneurysm method provides the most accurate aneurysm volume measurements when compared to the reference standard. It exhibited the highest proportional agreement, the smallest mean bias, and the strongest correlation coefficient. The semi-automated Philips Advanced Visualization Workspace also showed strong agreement, but to a lesser extent. The traditional 2D linear estimation method demonstrated the greatest underestimation and the weakest correlation with the reference standard.
DISCUSSION
This study compared three approaches for measuring aneurysm volume-traditional 2D linear estimation, a semi-automated 3D technique (Philips Advanced Visualization Workspace), and an AI-based 3D method (RapidAI for Aneurysm), against a reference standard of manual segmentation by experienced specialists. All three techniques underestimated aneurysm volumes to some degree compared to the reference standard.
Among the three methods, the traditional 2D linear estimation displayed the largest discrepancy, with a mean bias of –24.7 mm3 and a Passing-Bablok regression slope of 0.83. Its reliance on simplified geometry, such as ellipsoidal or cylindrical approximations, contributes to inaccuracies when dealing with irregularly shaped or multilobulated aneurysms. Although certain 2D-based formulas may sometimes overestimate volumes in specific cases,[
The semi-automated 3D technique using Philips software improved accuracy over the 2D method, with a mean bias of –19.5 mm3 and a Passing-Bablok slope of 0.87. This enhancement reflects the ability of 3D volumetric techniques to better account for complex geometries. However, variability persists due to clinician input during segmentation; operator-dependent adjustments and inconsistencies in defining the ROI can introduce errors, affecting both reliability and reproducibility. Despite this, semi-automated 3D techniques generally offer greater accuracy than traditional 2D methods.[
The AI-based 3D method using RapidAI for Aneurysm demonstrated the strongest correlation with the reference standard, with a mean bias of –14.5 mm3 and a Passing-Bablok slope of 0.94. Despite its superior performance, it still underestimated aneurysm volumes, although to a lesser extent. This contrasts with some findings where AI systems tend to overestimate volumes compared to manual assessments.[
These findings underline the importance of adopting precise, reproducible volumetric methods, whether for guiding coiling strategies or following aneurysms that have not yet reached a threshold for intervention. As imaging technologies progress, and as AI-based methodologies become more accurate and user-friendly, the potential to reduce unnecessary retreatments, improve the timing of interventions, and ultimately lower the risk of rupture appears increasingly within reach.
Future directions
Advancements in AI and machine learning hold significant potential to enhance aneurysm volume measurement accuracy and efficiency. Refining AI-based tools could improve performance, potentially surpassing manual segmentation and facilitating real-time decision-making without prolonging procedures. Developing and validating AI-based volumetric tools using magnetic resonance imaging (MRI) is another promising avenue, as MRI is a noninvasive modality increasingly used for screening and follow-up. This could broaden the applicability of volumetric assessments and offer an alternative to invasive imaging techniques.
Standardizing volume measurement methods is essential to reduce variability and improve study comparability. Establishing consensus guidelines and providing structured training on advanced software tools will ensure consistency and reliability, ultimately advancing intracranial aneurysm management and improving patient outcomes.
Limitations
This study has several limitations. The relatively small sample size may limit the generalizability of our findings; larger cohorts are needed to validate these results across diverse aneurysm sizes, shapes, and locations. The reference standard of manual segmentation, while accurate, is time-consuming and may introduce interobserver variability, despite averaging measurements from multiple specialists to mitigate this issue. In addition, we focused on volume measurements without assessing the impact of these discrepancies on clinical outcomes such as recanalization or retreatment rates. Prospective studies correlating measurement accuracy with long-term patient outcomes would provide valuable insights into the clinical significance of these findings.
CONCLUSION
All methods underestimated aneurysm volumes compared with the manual reference standard, potentially leading to inflated packing density estimates and overlooking subtle changes in aneurysm size. Traditional 2D estimation showed the greatest inaccuracy, while the semi-automated and AI-based methods provided closer approximations, with RapidAI showing the strongest correlation. Although packing density remains one factor in treatment planning, more precise volumetric assessments-particularly those driven by AI-can improve early detection of aneurysm growth, optimize clinical decision-making, and support standardized follow-up protocols for both treated and conservatively managed aneurysms.
Ethical approval:
The research/study approved by the Institutional Review Board at Research Ethics Committee of the Hospital Israelita Albert Einstein and the National Research Ethics Commission, number CAAE: 39713520.0.0000.0071; Approval Number: 4.897.322, dated August 10, 2021.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bizjak Ž Špiclin Ž. Aneurysm growth evaluation and detection: A computer-assisted follow-up MRA analysis. Sci Rep. 2024. 14: 19609
2. Chien A, Callender RA, Yokota H, Salamon N, Colby GP, Wang AC. Unruptured intracranial aneurysm growth trajectory: Occurrence and rate of enlargement in 520 longitudinally followed cases. J Neurosurg. 2020. 132: 1017-87
3. Erhardt S, Marbacher S, Neuschmelting V, Coluccia D, Remonda L, Fandino J. Comparison between routine cylindrical cerebral aneurysm volume approximation and three-dimensional volume measurements in experimental aneurysms. Neurol Res. 2014. 36: 739-45
4. Ernst M, Buchholz A, Bourcier R, Desal H, Le Floch PY, Möhlenbruch M. Voxel based analysis of recurrence dynamics in intracranial aneurysms after coiling. J Neurointerv Surg. 2018. 10: 571-6
5. Fatania K, Patankar T. Comprehensive review of the recent advances in devices for endovascular treatment of complex brain aneurysms. Br J Radiol. 2022. 95: 20210538
6. Gondar R, Gautschi OP, Cuony J, Perren F, Jägersberg M, Corniola MV. Unruptured intracranial aneurysm follow-up and treatment after morphological change is safe: Observational study and systematic review. J Neurol Neurosurg Psychiatry. 2016. 87: 1277-82
7. Hassankhani A, Ghozy S, Biglin C, Kadirvel R, Kallmes DF. Packing density and the angiographic results of coil embolization of intracranial aneurysms: A systematic review and meta-analysis. Interv Neuroradiol. 2023. 12: 15910199231155288
8. Hoh BL, Ko NU, Amin-Hanjani S, Chou SH, Cruz-Flores S, Dangayach NS. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: A guideline from the American heart association/American stroke association. Stroke. 2023. 54: e314-70
9. Johnston SC, Higashida RT, Barrow DL, Caplan LR, Dion JE, Hademenos G. Recommendations for the endovascular treatment of intracranial aneurysms: A statement for healthcare professionals from the committee on cerebrovascular imaging of the American heart association council on cardiovascular radiology. Stroke. 2002. 33: 2536-44
10. Liu X, Haraldsson H, Wang Y, Kao E, Ballweber M, Martin AJ. A volumetric metric for monitoring intracranial aneurysms: Repeatability and growth criteria in a longitudinal MR imaging study. AJNR Am J Neuroradiol. 2021. 42: 1591-7
11. Malhotra A, Wu X, Geng B, Hersey D, Gandhi D, Sanelli P. Management of small unruptured intracranial aneurysms: A survey of neuroradiologists. AJNR Am J Neuroradiol. 2018. 39: 875-80
12. Ou C, Qian Y, Chong W, Hou X, Zhang M, Zhang X. A deep learning-based automatic system for intracranial aneurysms diagnosis on three-dimensional digital subtraction angiographic images. Med Phys. 2022. 49: 7038-53
13. Pierot L, Barbe C, Herbreteau D, Gauvrit JY, Januel AC, Bala F. Immediate post-operative aneurysm occlusion after endovascular treatment of intracranial aneurysms with coiling or balloon-assisted coiling in a prospective multicenter cohort of 1189 patients: Analysis of recanalization after endovascular treatment of intracranial aneurysm (ARETA) study. J Neurointerv Surg. 2021. 13: 918-23
14. Piotin M, Daghman B, Mounayer C, Spelle L, Moret J. Ellipsoid approximation versus 3D rotational angiography in the volumetric assessment of intracranial aneurysms. AJNR Am J Neuroradiol. 2006. 27: 839-42
15. Planinc A, Špegel N, Podobnik Z, Šinigoj U, Skubic P, Choi JH. Assessing accuracy and consistency in intracranial aneurysm sizing: Human expertise vs. Artificial intelligence. Sci Rep. 2024. 14: 16080
16. Sahlein DH, Gibson D, Scott JA, Denardo A, Amuluru K, Payner T. Artificial intelligence aneurysm measurement tool finds growth in all aneurysms that ruptured during conservative management. J Neurointerv Surg. 2023. 15: 766-70
17. Takao H, Ishibashi T, Saguchi T, Arakawa H, Ebara M, Irie K. Validation and initial application of a semiautomatic aneurysm measurement software: A tool for assessing volumetric packing attenuation. Am J Neuroradiol. 2014. 35: 721-6
18. Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES. Guidelines for the management of patients with un ruptured intracranial aneurysms: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2015. 46: 2368-400
19. Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: Growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013. 269: 258-65