- Georgia Neurosurgical Institute, Macon, Georgia, USA
- Department of Neurosurgery, Yerevan State Medical University, Yerevan, Armenia
- Glendale Adventist Comprehensive Stroke Center, Los Angeles, California, USA
Correspondence Address:
Tigran Khachatryan
Georgia Neurosurgical Institute, Macon, Georgia, USA
DOI:10.4103/sni.sni_317_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tigran Khachatryan, Marina Khachatryan, Ruben Fanarjyan, Mikayel Grigoryan, Arthur Grigorian. Enlargement of an incidental internal carotid artery aneurysm embedded in pituitary adenoma associated with medical shrinkage of the tumor: Case report. 14-Feb-2018;9:30
How to cite this URL: Tigran Khachatryan, Marina Khachatryan, Ruben Fanarjyan, Mikayel Grigoryan, Arthur Grigorian. Enlargement of an incidental internal carotid artery aneurysm embedded in pituitary adenoma associated with medical shrinkage of the tumor: Case report. 14-Feb-2018;9:30. Available from: http://surgicalneurologyint.com/surgicalint-articles/enlargement-of-an-incidental-internal-carotid-artery-aneurysm-embedded-in-pituitary-adenoma-associated-with-medical-shrinkage-of-the-tumor-case-report/
Abstract
Background:Currently, transsphenoidal surgery (TSS) is the preferred method for surgical treatment of intrasellar pituitary adenomas. However, it carries some risk of intraoperative arterial injuries, which is mainly attributed to direct iatrogenic rupture of the internal carotid artery (ICA). There is anecdotal evidence suggesting that intracranial aneurysms are coincidentally found significantly more frequently in the setting of pituitary adenomas than when the incidence is compared to other intracranial neoplasms. The exact cause of this discrepancy remains unclear, but it certainly raises concerns about the potential existence of an ICA aneurysm, which might be encountered during TSS and in some cases may cause hemorrhagic complications.
Case Description:We present a case of a patient who was found to have a growth hormone (GH)-secreting pituitary adenoma and a coexisting cavernous ICA aneurysm which was embedded within the tumor. The patient underwent medical treatment of the adenoma. However, shrinkage of the tumor was associated with enlargement of the observed aneurysm, warranting endovascular intervention.
Conclusions:This case report is an illustration for physicians to be conscientious about the potential danger posed by the coexistence of an intratumoral aneurysm in the setting of a pituitary adenoma. Special attention should be given to recognition of an intrinsic flow void signal on the presurgical imaging of the tumor, and if observed, magnetic resonance angiography (MRA) should be performed for preoperative planning. If MRA is not performed routinely, detailed review of high-resolution magnetic resonance imaging is recommended to detect any flow artifacts suggestive of an aneurysm.
Keywords: Aneurysm, coincidence, complications, iatrogenic injury, intraoperative bleeding, pituitary adenoma, transsphenoidal
INTRODUCTION
Currently, microscopic or endoscopic transsphenoidal surgery (TSS) is the preferred method for the treatment of intrasellar pituitary adenomas.[
We present a case of a patient who was found to have a growth hormone (GH)-secreting pituitary adenoma and a coexisting cavernous ICA aneurysm embedded within the tumor. The patient underwent medical treatment of the adenoma. However, shrinkage of the tumor resulted in simultaneous enlargement of the observed aneurysm warranting endovascular intervention.
CASE REPORT
Initial management
A 37-year-old female presented to our department after noticing progressive enlargement of her jaw, ears, nose, and fingers. Physical examination and comparison to previous photographs confirmed acromegaly. Further neurological examination including formal visual field testing was within normal limits. A full spectrum of pituitary axis investigations was performed, which revealed increased levels of IGF-1 (1109 ng/ml) as well as increased levels of GH (15 ng/ml). All other hormonal laboratory results were within the normal reference range.
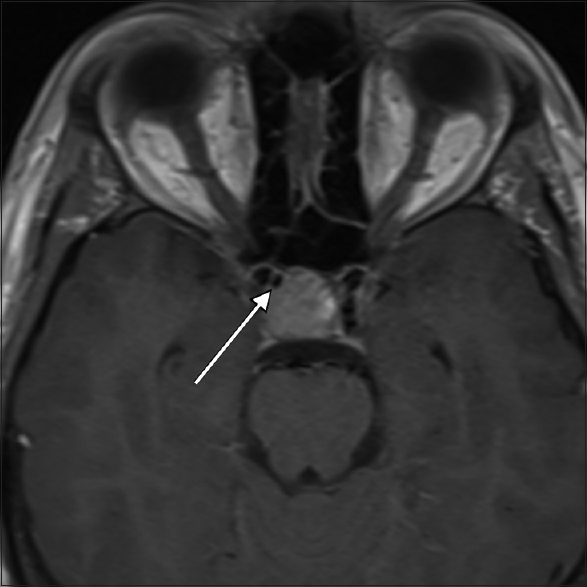
High-resolution magnetic resonance imaging (MRI) of the brain with contrast media was performed, which revealed a 17.5 mm × 15.7 mm × 17.4 mm pituitary macroadenoma with stalk displacement. The patient was admitted to our department for elective transsphenoidal surgery. During the preoperative review of MRI films, a subtle area of flow void was noticed within the right anterior aspect of the tumor [
The decision was made to perform a magnetic resonance angiography (MRA) for a more detailed visualization of the vasculature, which revealed a 2.5-mm cavernous ICA aneurysm pointing towards the sella.
A detailed discussion was then carried out with the patient about the natural history of adenomas and all available treatment options, including endovascular treatment of the aneurysm followed by TSS. We also discussed associated risks and benefits as well as the risks of leaving the adenoma untreated. The patient elected to first pursue medical treatment for pituitary adenoma with close follow-up for the aneurysm.
Continued management
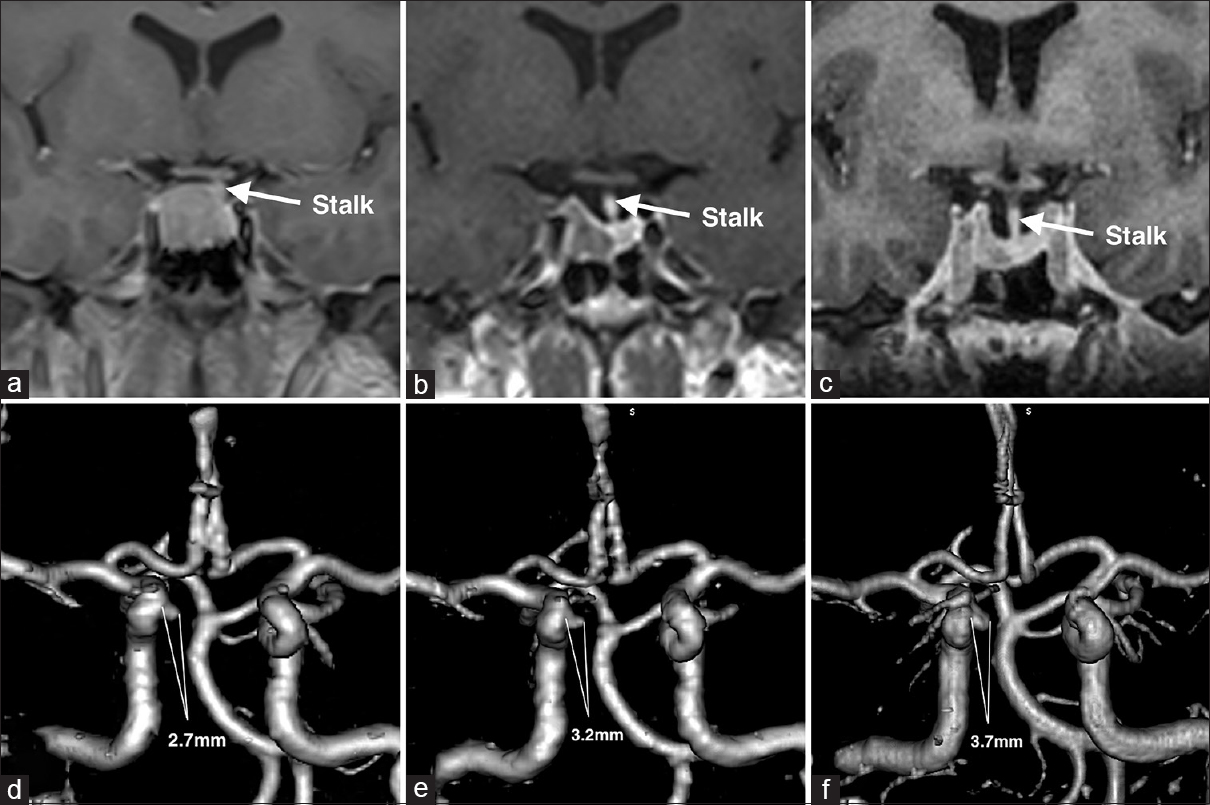
Shortly after initiation of monthly intramuscular injections of 30 mg of octreotide depot and twice a week administration of 0.25 mg oral cabergoline, the patient noticed a significant decrease in the soft tissue swelling of her face and extremities. IGF-1 levels showed a steady decline from 1109 ng/ml to 353.1 ng/ml over the follow-up period of 1.5 years. The efficacy of medical therapy was also confirmed by a steady decline in tumor size from 17.5 mm × 15.7 mm × 17.4 mm to 10.9 mm × 4.9 mm × 8.9 mm [Figure
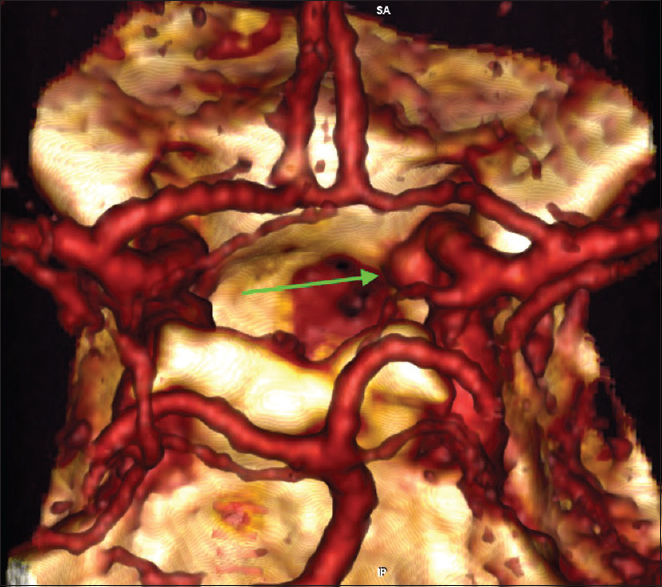
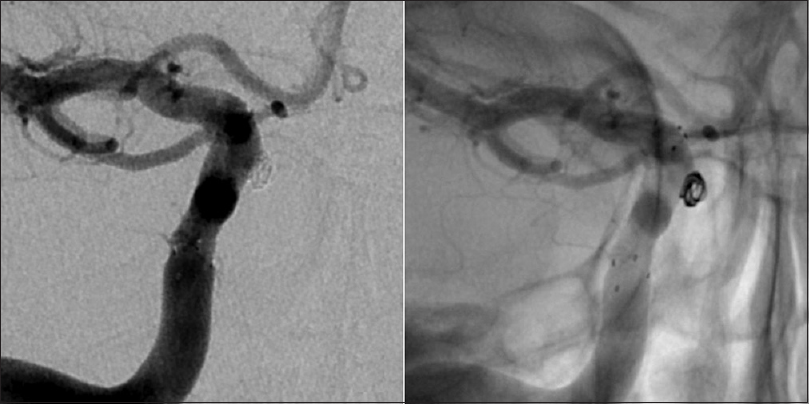
Six months later a CT angiography was performed at an outside hospital where the patient was seen for a second opinion [
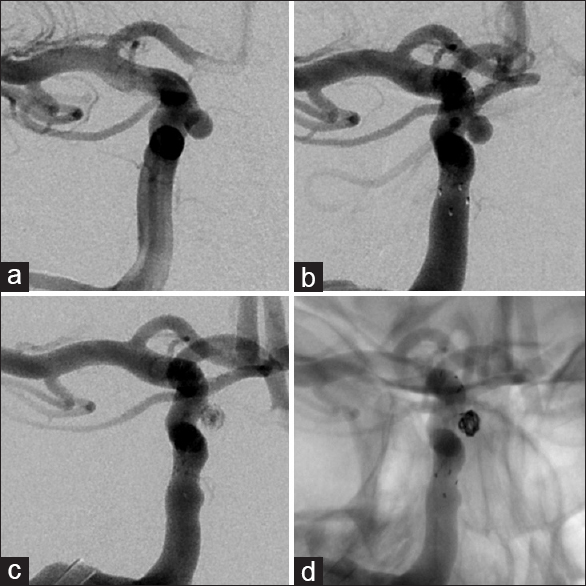
Given the steady increase in the size of the aneurysm and considering the absence of reliable data about the natural history of intratumoral aneurysms, the patient was again offered endovascular treatment and subsequently underwent a successful stent-assisted coil embolization of the aneurysm without complications [
DISCUSSION
Rupture of aneurysms located in the parasellar cavernous segment of the ICA occurs rarely. It is usually clinically silent though cranial nerve deficits have been reported in some cases.[
TSS is the preferred method of treatment for GH-secreting tumors, and medical therapy is indicated in cases when contraindications to surgery exist.[
There are several case reports about the development of a postoperative pseudoaneurysm of the ICA.[
According to Choi et al., incidental intracranial aneurysms are found seven times more frequently in pituitary adenomas than in other types of tumors.[
Finally, there is no reliable data about the natural history of coexisting aneurysms in the presence of GH-secreting pituitary adenomas. Spitler et al. have found a higher incidence in aneurysm growth rate in patients suffering from GH-secreting tumors.[
CONCLUSIONS
Single case reports do not provide enough evidence to draw scientific conclusions. However, they can serve as a reminder about potential perils in daily practice.
Eye-catching pathologies often draw attention away from other subtle coincidental findings. Neurosurgeons must remain alert about possible coincidental pathologies, which may lead to devastating intraoperative complications. Special attention should be devoted to recognition of a flow void within the tumor and MRA should preferably be performed during the preoperative planning. If MRA is not performed, detailed review of high-resolution MRI should be conducted to reveal any flow artifacts suggestive of an aneurysm. The association of tumor shrinkage and aneurysm enlargement in our case does not imply causality. A prospective multicenter screening study could reveal the true incidence of intrasellar aneurysms associated with pituitary adenomas and compare its natural history to that of other types of intracranial aneurysms.
Patient consent
The patient has consented to submission of this case report to the journal.
Financial support and sponsorship
Nil.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
1. Barker FG, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996-2000: Mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003. 88: 4709-19
2. Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002. 97: 293-8
3. Cavallo LM, Briganti F, Cappabianca P, Maiuri F, Valente V, Tortora F. Hemorrhagic vascular complications of endoscopic transsphenoidal surgery. Minim Invasive Neurosurg. 2004. 47: 145-50
4. Charalampaki P, Ayyad A, Kockro RA, Perneczky A. Surgical complications after endoscopic transsphenoidal pituitary surgery. J Clin Neurosci. 2009. 16: 786-9
5. Chen CC, Carter BS, Wang R, Patel KS, Hess C, Bodach ME. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Preoperative Imaging Assessment of Patients With Suspected Nonfunctioning Pituitary Adenomas. Neurosurgery. 2016. 79: E524-6
6. Choi HS, Kim MS, Jung YJ, Kim OL. Incidental Superior Hypophygeal Artery Aneurysm Embedded within Pituitary Adenoma. J Korean Neurosurg Soc. 2013. 54: 250-2
7. Chowdhury T, Prabhakar H, Bithal PK, Schaller B, Dash HH. Immediate postoperative complications in transsphenoidal pituitary surgery: A prospective study. Saudi J Anaesth. 2014. 8: 335-41
8. Cinar C, Bozkaya H, Parildar M, Oran I. Endovascular Management of Vascular Injury during Transsphenoidal Surgery. Interv Neuroradiol. 2013. 19: 102-9
9. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: Results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997. 40: 225-
10. Dolenc VV, Lipovsek M, Slokan S. Traumatic aneurysm and carotid-cavernous fistula following transsphenoidal approach to a pituitary adenoma: Treatment by transcranial operation. Br J Neurosurg. 1999. 13: 185-8
11. Eddleman CS, Surdell D, Miller J, Shaibani A, Bendok BR. Endovascular management of a ruptured cavernous carotid artery aneurysm associated with a carotid cavernous fistula with an intracranial self-expanding microstent and hydrogel-coated coil embolization: Case report and review of the literature. Surg Neurol. 2007. 68: 562-7
12. Ghatge SB, Modi DB. Treatment of ruptured ICA during transsphenoidal surgery. Two different endovascular strategies in two cases. Interv Neuroradiol. 2010. 16: 31-7
13. Guvenc G, Kizmazoglu C, Pinar E, Imre A, Kaya I, Bezircioglu H. Outcomes and Complications of Endoscopic Versus Microscopic Transsphenoidal Surgery in Pituitary Adenoma. J Craniofac Surg. 2016. 27: 1015-20
14. Halvorsen H, Ramm-Pettersen J, Josefsen R, Ronning P, Reinlie S, Meling T. Surgical complications after transsphenoidal microscopic and endoscopic surgery for pituitary adenoma: A consecutive series of 506 procedures. Acta Neurochir (Wien). 2014. 156: 441-9
15. Iancu D, Lum C, Ahmed ME, Glikstein R, Dos Santos MP, Lesiuk H. Flow diversion in the treatment of carotid injury and carotid-cavernous fistula after transsphenoidal surgery. Interv Neuroradiol. 2015. 21: 346-50
16. Kachhara R, Menon G, Bhattacharya RN, Nair S, Gupta AK, Gadhinglajkar S. False aneurysm of cavernous carotid artery and carotid cavernous fistula: Complications following transsphenoidal surgery. Neurol India. 2003. 51: 81-3
17. Kuo JS, Barkhoudarian G, Farrell CJ, Bodach ME, Tumialan LM, Oyesiku NM. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Surgical Techniques and Technologies for the Management of Patients With Nonfunctioning Pituitary Adenomas. Neurosurgery. 2016. 79: E536-8
18. Laws ER. Complications of transsphenoidal surgery: The shortcomings of meta-analysis. J Neurol Neurosurg Psychiatry. 2013. 84: 829-
19. Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E. Complications Related to the Endoscopic Endonasal Transsphenoidal Approach for Nonfunctioning Pituitary Macroadenomas in 300 Consecutive Patients. World Neurosurg. 2016. 89: 442-53
20. Moon K, Albuquerque FC, Ducruet AF, Crowley RW, McDougall CG. Resolution of cranial neuropathies following treatment of intracranial aneurysms with the Pipeline Embolization Device. J Neurosurg. 2014. 121: 1085-92
21. Nemergut EC, Dumont AS, Barry UT, Laws ER. Perioperative management of patients undergoing transsphenoidal pituitary surgery. Anesth Analg. 2005. 101: 1170-81
22. Onishi H, Ito H, Kuroda E, Yamamoto S, Kubota T. Intracranial mycotic aneurysm associated with transsphenoidal surgery to the pituitary adenoma. Surg Neurol. 1989. 31: 149-54
23. Oran I, Parildar M, Dalbasti T, Memis A, Ozdamar N. Intradural Aneurysm Caused by Arterial Injury during Surgery: Treatment with Coil Embolization. Interv Neuroradiol. 2001. 7: 353-6
24. Oyama H, Wada K, Hattori K, Kito A, Maki H, Noda T. [A case of pituitary adenoma in which epistaxis caused by the postoperative ruptured pseudoaneurysm of the sphenopalatine artery was treated by the endovascular embolization]. No Shinkei Geka. 2014. 42: 473-7
25. Padhye V, Valentine R, Wormald PJ. Management of carotid artery injury in endonasal surgery. Int Arch Otorhinolaryngol. 2014. 18: S173-8
26. Park KY, Kim BM, Kim DJ. Preoperative Coiling of Coexisting Intracranial Aneurysm and Subsequent Brain Tumor Surgery. Korean J Radiol. 2016. 17: 931-9
27. Peng Z, Tian D, Wang H, Kong DK, Zhang S, Liu B. Epistaxis and pituitary apoplexy due to ruptured internal carotid artery aneurysm embedded within pituitary adenoma. Int J Clin Exp Pathol. 2015. 8: 14189-97
28. Pereira Filho Ade A, Gobbato PL, Pereira Filho Gde A, Silva SB, Kraemer JL. Intracranial intrasellar kissing carotid arteries: Case report. Arq Neuropsiquiatr. 2007. 65: 355-7
29. Raikundalia MD, Pines MJ, Svider PF, Baredes S, Folbe AJ, Liu JK. Characterization of transsphenoidal complications in patients with acromegaly: An analysis of inpatient data in the United States from 2002 to 2010. Int Forum Allergy Rhinol. 2015. 5: 417-22
30. Shakir HJ, Garson AD, Sorkin GC, Mokin M, Eller JL, Dumont TM. Combined use of covered stent and flow diversion to seal iatrogenic carotid injury with vessel preservation during transsphenoidal endoscopic resection of clival tumor. Surg Neurol Int. 2014. 5: 81-
31. Spitler K, Drazin D, Hanna G, Patel A, Chu R. Association of intracranial aneurysms with meningiomas, pituitary adenomas, and gliomas: Review of possible interrelationships. ISRN Neurol. 2013. 2013: 383425-
32. Teramoto A. Contemporary transsphenoidal surgery for pituitary adenomas with emphasis on complications. Biomed Pharmacother. 2002. 56: 154s-7s
33. Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES. Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015. 46: 2368-400
34. van Rooij WJ, Sluzewski M, Beute GN. Ruptured cavernous sinus aneurysms causing carotid cavernous fistula: Incidence, clinical presentation, treatment, and outcome. AJNR Am J Neuroradiol. 2006. 27: 185-9
35. Wanke I, Doerfler A, Stolke D, Forsting M. Carotid cavernous fistula due to a ruptured intracavernous aneurysm of the internal carotid artery: treatment with selective endovascular occlusion of the aneurysm. J Neurol Neurosurg Psychiatry. 2001. 71: 784-
36. Yamada S, Yamada SM, Hirohata T, Ishii Y, Hoya K, Murakami M. Endoscopic extracapsular removal of pituitary adenoma: The importance of pretreatment of an adjacent unruptured internal carotid artery aneurysm. Case Rep Neurol Med. 2012. 2012: 891847-
37. Yoon KW, Cho CS, Lee SK. Large intracranial aneurysm after transsphenoidal surgery for pituitary macroadenoma. J Korean Neurosurg Soc. 2014. 55: 160-3
38. Zada G, Woodmansee WW, Iuliano S, Laws ER. Perioperative management of patients undergoing transsphenoidal pituitary surgery. Asian J Neurosurg. 2010. 5: 1-6
39. Zhou WG, Yang ZQ. Complications of transsphenoidal surgery for sellar region: Intracranial vessel injury. Chin Med J (Engl). 2009. 122: 1154-6