- Department of Neurosurgery, Faculty of Medical Sciences, Lebanese University, Lebanon
- Masters In Medical Ethics, Registered Nurse, Lebanese University, Lebanon
- Department of Pharmacology, Lebanese International University, Lebanon
- Department of Infectious Diseases, Lebanese University, Beirut, Lebanon
- Department of Pediatrics, Saint Georges Hospital, Hadath, Lebanon
- Department of General Medicine, Saint Joseph University, Beirut, Lebanon
- Department of General Medicine, Lebanese University, Lebanon
- Professor of Neurology, Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon
Correspondence Address:
Ali Naser Msheik, Department of Neurological Surgery, Lebanese University, Lebanon.
DOI:10.25259/SNI_457_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ali Naser Msheik1, Zeinab Al Mokdad2, Faten Hamed3, Farah Assi4, Ali Jibbawi5, Jean-Pierre Saad6, Rami Mohanna6, Anthony Khoury7, Mhamad Farhat6, Rami Atat8. Epstein–Barr virus flare: A multiple sclerosis attack. 04-Oct-2024;15:355
How to cite this URL: Ali Naser Msheik1, Zeinab Al Mokdad2, Faten Hamed3, Farah Assi4, Ali Jibbawi5, Jean-Pierre Saad6, Rami Mohanna6, Anthony Khoury7, Mhamad Farhat6, Rami Atat8. Epstein–Barr virus flare: A multiple sclerosis attack. 04-Oct-2024;15:355. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13137
Abstract
Background: Multiple sclerosis (MS)-Epstein–Barr virus (EBV) relation is similar to doing a complicated puzzle: it consists of many pieces that become more and more clear as the issue is viewed from different sides. Based on the research findings, there is powerful evidence that EBV and MS have a strong relation where high levels of EBV DNA are able to be shown in all the spinal cord and the blood of the MS patients, but these are shown during disease relapses, and this implies a role in these illnesses. It kind of narrows the choices that you have to look for, just like how gathering evidence can lead to finding the missing person. In the analysis, new ways of EBV participation in MS progression are expected to be installed, and even new therapeutics are expected to be made.
Methods: A comprehensive literature search of PubMed was conducted until November 2023 to identify studies investigating the association between Epstein-Barr virus (EBV) infection and multiple sclerosis (MS). Only articles that met stringent criteria, including validation of EBV infection through laboratory testing, were included in the analysis.
Results: A total of 16 articles were identified as applicable for the background review, and this conformed with the discovery that the initiation of EBV/IM was consistent across various studies, namely, retrospective, cross-sectional, or prospective. The statistics reveal a glimpse into the need for prolonged research in studying the pattern of this link between EBV and MS. Novel treatment approaches targeting EBV, including adoptive T-cell therapy and gene-based immunotherapy, show promise in mitigating MS progression by targeting EBV-infected cells.
Conclusion: Clinical trials investigating antiviral therapies and vaccination strategies are underway, aiming to translate these findings into effective treatments for MS. Despite promising advances, challenges remain in developing EBV-targeted therapies for MS, including safety concerns and the multifactorial nature of MS pathogenesis. Advance treatment options that focus on EBV, such as adoptive T-cell therapy and gene-based immunotherapy, are shown to be effective in the improvement of MS management that targets the viral-infected cell. The clinical trials for antiviral drugs and vaccination tactics are going on to benefit from these findings and eventually to invent effective therapeutics for MS. While these new therapeutic directions may offer great promise, challenges remain in these approaches as safety concerns and complex factors that underlie MS pathology need to be taken care of. The ethical aspects linked to picking the patients and giving informed consent make the progress of EBV-related treatments are even more difficult. Future research is recommended so that the primary mechanisms through which EBV contributes to MS development will be elucidated; in addition, the main MS subtype sources must be addressed. Longitudinal studies and other advanced research technologies will provide hope because they can solve the complicated problems of MS due to viruses and look for new therapeutic targets. The review brings up EBV/IM disease as a vital aspect of MS susceptibility, encouraging research in the field of longitudinal studies. Although we have made advances, we are still far from clear on the labyrinthine pairing between EBV and MS and the development of therapeutic strategies to attack EBV infection in MS patients.
Keywords: Chronic infection, Epstein–Barr virus, Flare, Multiple sclerosis, Neurological disease, MS, EBV, FLARE, Neurological Disease, Chronic infection
INTRODUCTION
The association between multiple sclerosis (MS) and Epstein– Barr virus (EBV) infection is essential for understanding the etiology of MS. This relationship is evidenced by various studies, including meta-analyses that demonstrate a significant link between EBV and MS and laboratory reports that detect EBV-infected cells in MS cases, particularly those identified through asymptomatic EBV infections. Research indicates elevated levels of EBV DNA in the spinal fluid and blood of MS patients, suggesting a contributory role of EBV in MS relapses. These findings illustrate patterns of EBV infection and MS prevalence, highlighting differential risk exposures across populations. Early indicators, such as increased neurofilament levels, offer predictive insights into MS progression. Reviewing these components provides a comprehensive understanding of EBV’s role in MS, guiding the development of novel therapeutic approaches. The study aims to examine EBV/infectious mononucleosis (IM) as an independent risk factor for MS onset instead of considering only the link between the risk of MS onset with the other factors. This can be done using advanced statistical analysis methods. Perfectly in line with Preferred Reporting Items for Systematic Reviews and Meta-Analysis protocol then, all the choices are standardized, which can be evaluated, in the end, as more clear and transparent.
Objectives
The objective of this research is to elucidate the role of EBV or infectious mononucleosis (IM) in the pathogenesis of MS. It is imperative to establish EBV/IM as an independent risk factor, potentially manifesting at any stage of a patient’s life or over an extended period. The objectives include examining the temporal relationship between EBV/IM infection and the onset of MS and excluding studies that do not meet established criteria, such as availability of full text, relevance to the primary objective, or accurate presentation of the temporal association between EBV/IM infection and MS onset.
REVIEW
Methodology
Search strategy
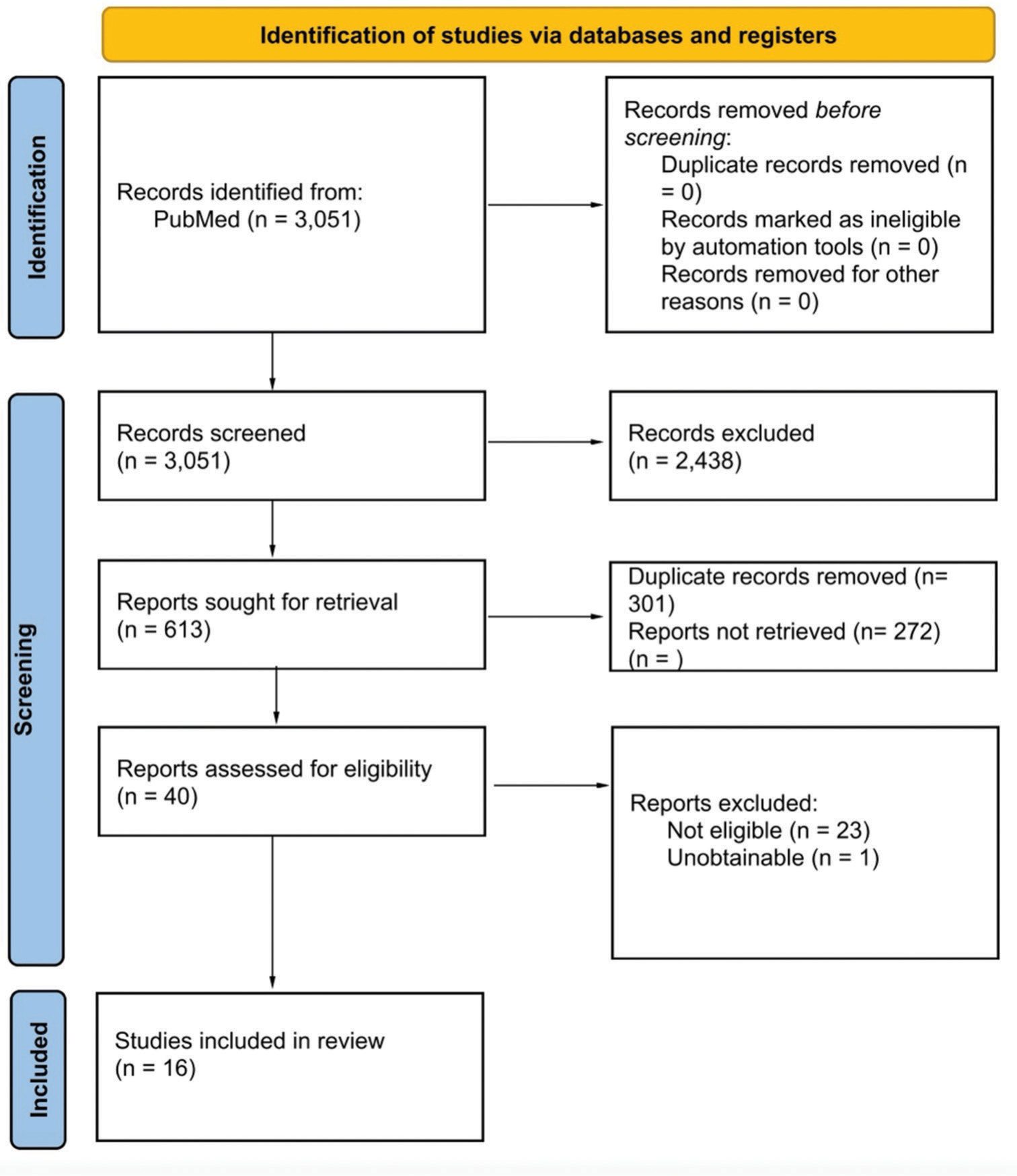
Our search on the PubMed database was performed till November 2023, looking for papers that claim that it may be the risk factor of MS in the endpoint previous EBV/IM. The scan was completed without date or language restrictions and any subject restrictions; it involved all fields of knowledge. The used search terms included the following: “MS,” “EBV,” and “MS occurred, which is not a single form of the disease since there are different types of diseases manifesting in the form of MS. Thus, EBV is a member of the human herpesvirus (HHV)-4 family also known as HHV-4. IM, which is referred to as IM as well as EBV, has some kind of search phrases, and their titles and abstracts were scrutinized for relevance; the matching papers were screened according to the inclusion criteria, and the outputs of the papers were saved. We channeled the process of selection in accordance with the Preferred Reporting Items for Systematic Reviews and the Meta-Analysis diagram found in
Eligibility criteria
The following eligibility criteria were applied in the review of retrieved articles: studies that are aimed at evaluating EBV/IM as an independent risk factor for the occurrence of MS across a longitudinal time frame, studies that prospectively or retrospectively assess the risk of MS in relation to EBV/IM in a temporal relationship or a reverse temporal relationship, IM/EBV and MS have been documented according to a verified laboratory parameter or McDonald’s/Poser criteria, and full-text availability. Articles not meeting the above criteria, studies with a main objective other than evaluating the risk of MS as a function of EBV infection, studies that are cross-sectional or do not retrospectively or prospectively follow the previously mentioned relationship in a temporal frame, articles that report EBV/IM infection through a validated or a self-reported questionnaire, reviews, systematic reviews, and case reports were excluded from the study.
Quality assessment
The included articles were of either two types: Case–controls or cohorts. After selecting the final included articles, the Newcastle-Ottawa Scale (NOS) tool was used to evaluate the quality of the articles. This tool assesses the quality of published articles using an asterisk scoring system with respect to three parameters: selection, comparability, and outcome (cohorts) or exposure (case–control studies). In the articles selected, the highest score was 8, and the lowest was 5. The average NOS score was 6.9.
RESULTS
A total of 16 articles[
The choice of the main subjects was mainly based on the variety of registries that exist worldwide [
There were just four articles that provided the mechanism of action from the exposure of EBV or IM to the time of MS disease onset. Three of those articles[
DISCUSSION
Data and results from the literature can be sorted into several subtopics. Below is a detailed discussion of each topic.
Epidemiological insights into the EBV-MS connection
MS is dominated by a number of risk factors, which include infection by EBV and such discovered association is considered a major element. An unfathomably vast meta-analysis has unearthed an association between the virus EBV and MS in as many as 96% of the included studies. It was 2011 when the most asked question for the profession was the reliability of correlation. This was answered when the B-cells of EBV-infected samples were treated in 21 out of 22 MS patients and were absent in other inflammatory neurological diseases.[
In the relapsing-remitting MS (RRMS) study, RNA-precipitation analysis was used to analyze EBV nuclear antigen (EBNA)-1 DNA in cerebrospinal fluid (CSF) and blood samples, with higher viral loads in RRMS patients than in controls.[
The distinct EBV’s role elicited in the MS early phase was significantly associated with a biomarker of neurofilaments that presented an increase 6 years before MS attack commencement while at the same time giving room for further exploration of other central nervous system biomarker changes, especially in the neurodegenerative disorder. An EBV infection of MS suffered patients was statistically demonstrated to rise following an EBV infection. This strongly revealed a possibility of a close and immunological mimicry mechanism. It is EBV and driven-interleukin-23 release and the activation mechanism of proinflammatory memory B-cells that become prominent in the disease progression within the central nervous system.[
Recent developments: Exploring EBV and MS relationship with novel methodologies
The most common techniques for studying the role of EBV in MS are observational studies that show higher levels of EBV in MS patients and assays such as enzyme-linked immunosorbent assay flow cytometry and genome-wide association studies. Studies that followed were focused on getting a grip on how the virus interacted with the disease to enable proper patient care. To pinpoint the certain transcription factors involved highly in the disease-related risk loci, Harley et al. developed the regulatory element locus intersection (RELI) algorithm.[
The researchers from Hassani et al. focused on producing a high level of quality analysis by relying on a few hundred brain specimens (122 MS and non-MS cases) to identify the existence of EBV at the tissue level.[
Sadam et al. implemented a hypothesis-free method based on multivalent antigen variants analysis (MVVD) to get antibody epitope profiles of patients with optic neuritis (ON), either those who progress to MS or who did not and discovered new epitope biomarkers for assessing ON progression to MS using blood samples.[
Smith et al. used the transcriptome analyses of resting B-cells and two types of B at 7 days after EBV infection or CD40L/interleukin-4 stimulation to explore the B-cell activation pathway.[
Using their expertise, Soldan et al. and Lieberman et al. studied the molecular techniques that could influence the process of B-cells binding with BBB and subsequently penetrating the BBB mediating B-cell neuroinvasion.[
Keane et al., using an allele-specific chromatin immunoprecipitation PCR (allele-specific ChIP-PCR), analyzed 6 MS risk single nucleotide polymorphisms (SNPs) and observed that EBNA2 binding, based on the presence of the risk or protective allele, was discovered at five of these loci.[
Although Ristori’s crew member, by targeting the available recently mapped TT (TT) mainly came from the intergenic and intronic regions of half-life only minutes, have proven that the genomic region coding for TT is highly enriched with GWAS variants associated with MS.[
The human microbiota plays a key role in health and disease. Microbiomes are a dynamic ecosystem that consists of organisms living in symbiotic relationships. These relationships can be either neutral, beneficial, or harmful. Microbiomes can be found in different environments, such as the skin, gut, and lungs. While studying the two-chain-recombined B-cell Receptor (BCR) repertoire from blood and Cerebrospinal fluid of Multiple Sclerosis (CSF of MS) patients, it was discovered that the mAB MS39p2w174 is an immunoglobulin heavy-chain variable region gene (IGHV)-3-7 derivative, cross-reactions both the Epstein–Barr virus (EBV) nuclear antigen 1 (EBNA1) and Glial cell adhesion molecule (CAM). The complex structure of EBNA1 peptide epitope in combination with the MS39p2w174 Fab indicated that the Complementarity-determining region (CDRs) of all domains, except for Light Chain-Complementarity-determining region(LC-CD)R2, were in close contact with that region of EBNA1 which carries P394–P398 amino acids residues. It was also illustrated by peptide motif analysis of MS39p2w174, which revealed a Pro/Arg-rich motif that was quite similar to the central epitope in EBNA1 (AA395-AA399), confirming their interaction. Tissue-specific protein arrays, HuProt, were used that contain over 80% of the human proteome. Furthermore, GlialCAM (the chronic-active plaque of MS lesions) was revealed to be a critical binding partner with the MS39p2w174 (an MS candidate gene). BLI showed that the ancestor (germline) that unmutated (MS39p2w174) binds EBNA1 with about the same affinity as MS39p2w174.[
Exploring potential treatments: EBV-MS connection
The relationship between EBV and MS has turned out to be a search for therapy methods aimed at EBV. The current research considerations thoroughly explore the possibilities of targeting EBV in MS treatment, with adoptive T-cell therapy being one way of approaching the issue. This therapy focuses mainly on eliminating EBV-infected autoreactive B-cells, which contribute to the development of MS. It is based on the idea of reducing the EBV infection rate or virus load, thus reducing MS risk. We can apply another strategy focusing on improving the treatment ideology of MS that stops the main reason for the disease rather than its late factors. Considering the possibility of curing EBV disease through antiviral treatments that may slow disease progression and bring about the desired outcome is the objective of this approach. This is specifically so for the antiviral medicines comprising acyclovir and valacyclovir since the latter has received criticism for the ability to curtail EBV replication. The hoped result is the prevention or slowing down of MS progressing, or at least the reduction of risk factors for disease development.[
In addition, the third therapeutic strategy with proven effectiveness involves gene-based immunotherapy, in which cells targeted against EBV-infected malignant cells are administrated. The immunotherapeutic approach has also been under research predominated by B-cell depletion therapy (e.g., rituximab and ocrelizumab) that appear to be able to address B-cells, which perform the core function in EBV infection as well as MS. This multidimensional approach highlights the common purpose of developing those specific therapies against MS through understanding the regular dynamics between EBV and disease.
Clinical trials: EBV-targeted therapies for MS
Clinical studies are still independent and are mainly employing some antiviral agents, vaccination strategies, and cell-based interventions in patients with MS. The outcomes of these analyses are substantial in explaining EBV’s modality in terms of being both a cause and a consequence of illnesses like systemic lupus erythematosus.[
Challenges and limitations: Targeting EBV in MS patients
The medications directed against EBV in MS patients’ development become a formidable task due to the side effects of the ongoing EBV infection, that is, cancer hazards, autoimmune disorders, and other serious illnesses in humans.[
Besides that, the fact that the multifactorial etiology of MS is also the basis of the difficulties and limitations in the conceptualization of EBV-directed therapies for the disease is also a feature. MS is manifested as a multifaceted disease, with both genetics and environmental factors playing major roles. Indeed, the efficacy of EBV as a single causative factor is questionable in the face of numerous players interacting in a highly complex system, as it is rather difficult to identify this sole contributor. Notwithstanding, safety issues are critical because the immune system can be disconnected from its natural state, or antiviral drugs can be administered, resulting in unwanted and even more severe reactions. Furthermore, MS shows profound diversity among its “classes” and “manifestations,” which involve distinguishable patterns and progression. Although interventions that have been successful in one patient population cannot be generalized to all categories, they may still offer an optimistic prognosis for some. Moreover, the small number of mono-specific MS patients in EBV-targeted therapies and the lack of evidence on the long-term safety and efficacy remain a challenge.[
Ethical considerations: EBV-RELATED TREATMENTS for MS
Issues of a moral nature in the course of introducing the body environment virus as a treatment of MS include the meaning of selection and informed consent. Likewise, if interventions aimed at EBV-infected individuals, including antiviral treatments, were found to improve disease course or halt their progression, then we would have a clear identification of individuals who are highly vulnerable to MS post-EBV infection. The possibility for primary prevention among patients who are considered high-risk individuals is opened up as a result.[
Gaps and future directions
Present research about the role of EBV in MS would reveal that there are certain areas where our understanding is still lacking. The fact that the presence of EBV is 90%. Meanwhile, the specific mechanisms by which EBV contributes to MS pathogenesis are unknown and still emerge in clinical practice.[
Moreover, a number of the gaps in current research are related to the subject of the B-cell neuroinvasion on MS progression.[
The therapeutic approach for MS treatment in regard to EBV virus might be to activate the lytic phase of EBV in B-cells, then to use an antiviral drug, with trials using tenofovir and other antivirals likely to be used. At present, trials are in progress concerning the approaches of the EBV-targeted T-cell immunotherapy, mRNA vaccines developed against the EBV, and the clinical investigations on the antiviral therapies’ effects on EBV replication. Supplied by Massachusetts General Hospital, a Truvada therapeutic interventional clinical trial examines the effect of the drug on EBV levels in people with MS.[
The next study is undertaken by Queen Mary University London in which the impact of famciclovir on EBV activity in RRMS patients is analyzed, and Atara Biotherapeutics conducts a trial to investigate the safety and efficacy of ATA188, which is an allogeneic T-cell immunotherapy in PPMS and SPMS patient groups.[
CONCLUSION
This systematic review clarifies the complex connection between patients with previously diagnosed EBV infection or IM and their increased risk of developing MS. Through an extensive literature review and a thorough analysis of eligible articles with rigorous methods, we have emphasized a possible role of EBV/IM infection as one of the contributing factors of MS susceptibility. Our results strongly suggest that the EBV/IM relationship should be taken as the critical factor in the context of MS development, which provides an argument for the longitudinal studies to clarify the disease progression further. Although our systematic review highlights virtually all the connections between EBV and MS, additional studies are necessary to decipher the main trigger factors. Besides that, the identification of drugs targeting EBV may pave the way for the successful management or elimination of MS in such individuals.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alfredsson L, Olsson T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb Perspect Med. 2019. 1: a028944
2. Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernán MA, Olek MJ. Epstein-Barr virus antibodies and risk of multiple sclerosis: A prospective study. JAMA. 2001. 286: 3083-8
3. Bar-Or A, Pender MP, Khanna R, Steinman L, Hartung HP, Maniar T. Epstein-Barr virus in multiple sclerosis: Theory and emerging immunotherapies. Trends Mol Med. 2020. 26: 296-310
4. Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022. 375: 296-301
5. Cocuzza CE, Piazza F, Musumeci R, Oggioni D, Andreoni S, Gardinetti M. Quantitative detection of Epstein-Barr virus DNA in cerebrospinal fluid and blood samples of patients with relapsing-remitting multiple sclerosis. PLoS One. 2014. 10: 94497-10
6. De Jager PL, Chibnik LB, Cui J, Reischl J, Lehr S, Simon KC. Integration of genetic risk factors into a clinical algorithm for multiple sclerosis susceptibility: A weighted genetic risk score. Lancet Neurol. 2009. 8: 1111-9
7. Décard BF, von Ahsen N, Grunwald T, Streit F, Stroet A, Niggemeier P. Low vitamin D and elevated immunoreactivity against Epstein-Barr virus before first clinical manifestation of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012. 83: 1170-3
8. DeLorenze GN, Munger KL, Lennette ET, Orentreich N, Vogelman JH, Ascherio A. Epstein-Barr virus and multiple sclerosis: Evidence of association from a prospective study with long-term follow-up. Arch Neurol. 2006. 63: 839-44
9. Downham C, Visser E, Vickers M, Counsell C. Season of infectious mononucleosis as a risk factor for multiple sclerosis: A UK primary care case-control study. Mult Scler Relat Disord. 2017. 17: 103-6
10. Effects of antiviral therapies on Epstein-Barr virus replication; (2023-2024). Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05957913.
11. Famciclovir in multiple sclerosis; (2023-2024). Available from: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/famciclovir-inmultip.
12. Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Multiple sclerosis after infectious mononucleosis: Record linkage study. J Epidemiol Community Health. 2004. 58: 1032-5
13. Grut V, Biström M, Salzer J, Stridh P, Lindam A, AlonsoMagdalena L. Free vitamin D3 index and vitamin D-binding protein in multiple sclerosis: A presymptomatic case-control study. Eur J Neurol. 2022. 29: 2335-42
14. Guan Y, Jakimovski D, Ramanathan M, WeinstockGuttman B, Zivadinov R. The role of Epstein-Barr virus in multiple sclerosis: From molecular pathophysiology to in vivo imaging. Neural Regen Res. 2019. 14: 373-86
15. Haahr S, Koch-Henriksen N, Møller-Larsen A, Eriksen LS, Andersen HM. Increased risk of multiple sclerosis after late Epstein-Barr virus infection: A historical prospective study. Mult Scler. 1995. 1: 73-7
16. Harley JB, Chen X, Pujato M, Miller D, Maddox A, Forney C. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat Genet. 2018. 50: 699-707
17. Hassani A, Corboy JR, Al-Salam S, Khan G. Epstein-Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells. PLoS One. 2018. 13: e0192109
18. Hedström AK. Risk factors for multiple sclerosis in the context of Epstein-Barr virus infection. Front Immunol. 2023. 24: 1212676
19. Jons D, Grut V, Bergström T, Zetterberg H, Biström M, Gunnarsson M. Seroreactivity against lytic, latent and possible cross-reactive EBV antigens appears on average 10 years before MS induced preclinical neuroaxonal damage. J Neurol Neurosurg Psychiatry. 2023. 95: 325-32
20. Keane JT, Afrasiabi A, Schibeci SD, Swaminathan S, Parnell GP, Booth DR. The interaction of Epstein-Barr virus encoded transcription factor EBNA2 with multiple sclerosis risk loci is dependent on the risk genotype. EBioMedicine. 2021. 71: 103572
21. Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature. 2022. 603: 321-7
22. Lanz TV, Robinson WH, Ho PP, Steinman L. Roadmap for understanding mechanisms on how Epstein-Barr virus triggers multiple sclerosis and for translating these discoveries in clinical trials. Clin Transl Immunology. 2023. 12: e1438
23. Levin LI, Munger KL, Rubertone MV, Peck CA, Lennette ET, Spiegelman D. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005. 293: 2496-500
24. Lindberg C, Andersen O, Vahlne A, Dalton M, Runmarker B. Epidemiological investigation of the association between infectious mononucleosis and multiple sclerosis. Neuroepidemiology. 1991. 10: 62-5
25. Loosen SH, Kostev K, Jördens MS, Luedde T, Roderburg C. Overlap between irritable bowel syndrome and common gastrointestinal diagnoses: A retrospective cohort study of 29,553 outpatients in Germany. BMC Gastroenterol. 2022. 5: 48
26. Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaëlsson K, Neovius M. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016. 31: 125-36
27. Lünemann JD, Tintoré M, Messmer B, Strowig T, Rovira A, Perkal H. Elevated Epstein-Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann Neurol. 2010. 67: 159-69
28. Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009. 10: 1543-50
29. Munger KL, Levin LI, O’Reilly EJ, Falk KI, Ascherio A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: A prospective study among United States military personnel. Mult Scler. 2011. 17: 1185-93
30. Nielsen TR, Rostgaard K, Nielsen NM, Koch-Henriksen N, Haahr S, Sørensen PS. Multiple sclerosis after infectious mononucleosis. Arch Neurol. 2007. 64: 72-5
31. Phase 1/2 study to evaluate the safety and efficacy of ATA188 in subjects with progressive multiple sclerosis; (2023-2024). Available from: https://classic.clinicaltrials.gov/ct2/show/NCT03283826.
32. Rang X, Liu Y, Wang J, Wang Y, Xu C, Fu J. Identification of multiple sclerosis-related genes regulated by EBV-encoded micrornas in B cells. Mult Scler Relat Disord. 2022. 59: 103563
33. Sadam H, Pihlak A, Jaago M, Pupina N, Rähni A, Toots M. Identification of two highly antigenic epitope markers predicting multiple sclerosis in optic neuritis patients. EBioMedicine. 2021. 64: 103211-10
34. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish national patient registry: A review of content, data quality, and research potential. Clin Epidemiol. 2015. 7: 449-90
35. Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007. 26: 2899-12
36. Smith C, Khanna R. Adoptive T-cell therapy targeting Epstein-Barr virus as a treatment for multiple sclerosis. Clin Transl Immunol. 2023. 12: e1444
37. Smith N, Tierney R, Wei W, Vockerodt M, Murray PG, Woodman CB. Induction of interferon-stimulated genes on the IL-4 response axis by Epstein-Barr virus infected human b cells; relevance to cellular transformation. PLoS One. 2013. 27: 64868
38. Soldan SS, Lieberman PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. 2023. 21: 51-64
39. Soldan SS, Su C, Lamontagne RJ, Grams N, Lu F, Zhang Y. Epigenetic plasticity enables CNS-trafficking of EBV-infected B lymphocytes. PLoS Pathog. 2021. 9: 1009618
40. Tenofovir alafenamide for treatment of symptoms and neuroprotection in relapsing remitting multiple sclerosis; (2023-2024). Available from: https://classic.clinicaltrials.gov/ct2/show/NCT04880577.
41. Trial to assess the safety and feasibility of adoptive cell therapy with autologous EBV-specific cytotoxic T lymphocytes (CTL) in patients with a first clinical episode highly suggestive of multiple sclerosis; (2023-2024). Available from: https://classic.clinicaltrials.gov/ct2/show/NCT02912897.
42. Umeton R, Bellucci G, Bigi R, Romano S, Buscarinu MC, Reniè R. Multiple sclerosis genetic and non-genetic factors interact through the transient transcriptome. Sci Rep. 2022. 9: 7536-10
43. Xu Y, Hiyoshi A, Smith KA, Piehl F, Olsson T, Fall K. Association of infectious mononucleosis in childhood and adolescence with risk for a subsequent multiple sclerosis diagnosis among siblings. JAMA Netw Open. 2021. 1: e2124932