- Department of Postgraduate Surgical Studies, Royal College of Surgeons in Ireland, Dublin, Ireland
- Department of Surgery, Tallaght University Hospital, Dublin, Ireland.
Correspondence Address:
AbdelSalam Nedal Al-Sousi, Department of Postgraduate Surgical Studies, Royal College of Surgeons in Ireland, Dublin, Ireland.
DOI:10.25259/SNI_109_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: AbdelSalam Nedal Al-Sousi1, Maria C. Whelan2, Zahra Khalaf1. Evaluating intraoperative ultrasound (IOUS) in focal cortical dysplasia (FCD) resection surgery: A systematic review. 17-May-2024;15:165
How to cite this URL: AbdelSalam Nedal Al-Sousi1, Maria C. Whelan2, Zahra Khalaf1. Evaluating intraoperative ultrasound (IOUS) in focal cortical dysplasia (FCD) resection surgery: A systematic review. 17-May-2024;15:165. Available from: https://surgicalneurologyint.com/surgicalint-articles/12897/
Abstract
Background: Surgery is the best approach to treating focal cortical dysplasia (FCD)-related epilepsy; yet, it has suboptimal outcomes because distinguishing the boundaries between the FCD region and normal brain tissue intraoperatively poses a challenge. The use of intraoperative ultrasound (IOUS) helps demarcate FCD lesion borders leading to more accurate intraoperative resection. In this review, the use of IOUS for the resection of FCD was evaluated.
Methods: This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The Medline, Embase, Cochrane Library, Scopus Library, and Dynamed Library databases were searched, and two independent reviewers examined the articles. The search terms related to “drug-resistant epilepsy” and “intraoperative ultrasound.” The results between January 2008 and April 2022 were abridged for FCD type, ultrasound resolution, extent of lesion resection, correction of brain shift, postoperative neurological deficits, and postoperative seizure freedom (Engel classification).
Results: Ten articles were included in the study. The parameters used to assess the efficacy of IOUS in FCD surgery were ultrasound resolution, demarcation of lesion boundaries, correction of brain shift, postoperative neurological deficits, and seizure freedom. Most studies have shown that IOUS produces high-resolution images. Surgery for Type 2 FCD patients had better outcomes than surgery for Type 1 FCD patients due to better visualization by IOUS. Patients were classified as Engel class 1 or class 2 postoperatively. Eight studies found that IOUS was superior to magnetic resonance imaging in brain shift correction.
Conclusion: The preliminary results look promising, especially for the international league against epilepsy class 2 FCD. However, there is a need for more high-quality research evaluating the use of IOUS in FCD and comparing it to other intraoperative imaging modalities.
Keywords: Drug-resistant epilepsy, Epilepsy surgery, Focal cortical dysplasia (FCD), Intraoperative ultrasound (IOUS)
INTRODUCTION
Epilepsy is a chronic, non-communicable neurological condition characterized by recurrent unprovoked seizures.[
FCD was first described by Taylor et al. in 1971 as “congregations of large, bizarre neurons which were littered through all but the first cortical layer;”[
Since FCD-related epilepsy is typically of the drug-refractory type, surgery proves to be the best alternative currently, leading to postoperative seizure control in up to 80% of the patients if the lesion is completely resected.[
The most important prognostic factor of surgical outcome in FCD-related epilepsy patients is the completeness of lesion resection. [
Tools have been developed for intraoperative use to improve surgical outcomes for FCD. Imaging navigation systems with multiple fusion strategies, intraoperative MRI (IoMRI), and electrocorticography (ECoG), have proven very useful in facilitating the complete resection of FCD lesions, although they also come with inherent drawbacks.[
Intraoperative ultrasound (IOUS), on the other hand, is easily accessible and inexpensive, allows real-time 3-dimensional visualization without the need for long periods of surgery interruption, clearly displays adjacent vasculature, allows postsurgical cavity exploration, and is very reliable in demarcating lesions borders even with FCD.[
Despite the reported poor success rate of FCD-related epilepsy surgery due to inadequate removal of the lesion as a consequence of unsatisfactory intraoperative imaging, only a few papers discuss ways to improve surgical technique and intraoperative imaging. Although IOUS is not a novel tool in neurosurgery and has proven beneficial in facilitating the resection of many brain lesions, such as tumors and arteriovenous malformations,[
This review was conducted to systematically review recent papers about the implications of using ultrasound intraoperatively to facilitate the resection of FCD lesions in terms of outcome, success rate, and seizure control, if proven beneficial, to encourage standardizing its utilization among all relevant centers. Furthermore, in this review, we examined the potential for future research in this area.
MATERIALS AND METHODS
The presented systematic review was conducted in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols 2020.[
Inclusion and exclusion criteria
The inclusion criteria were as follows: Articles published between January 2008 and April 2022 in the English language and involving at least one human participant (e.g., case report). Publications were excluded if they were not published with a full text (e.g., conference abstracts), involved only subjects with causes of epilepsy other than FCD (e.g., intra-axial solid tumors), involved the use of only non-IOUS imaging modalities, or had a surgical purpose that was not a laminectomy (e.g., depth electrode insertion).
Search strategy
The search for potentially relevant publications was employed on October 1, 2022, utilizing five bibliographic databases and registries (MEDLINE, EMBASE, COCHRANE, SCOPUS, and DYNAMED). Details of the MEDLINE and EMBASE search strategies are presented in
Data extraction and reporting
A table was created to chart the data. The data were abridged for the number of patients, type of FCD, ultrasound resolution, completion of lesion resection (postoperative MRI scans), mention of IOUS’s value in the correction of brain shift, postoperative neurological deficits, and postoperative seizure freedom using the Engel classification system. To the best of our knowledge, no systematic reviews on this topic have been published. We intended to combine the data mentioned above for statistical analysis through a meta-analysis. However, due to the large heterogeneity of the data, a meta-analysis was found to be unsuitable, so we proceeded with a qualitative synthesis for the description of the data instead. Furthermore, a quantitative synthesis in the form of frequencies, percentages, and ranges was used to describe the cases of US-detected FCD and seizure freedom.
Assessment of the quality of evidence
A total of ten studies were included in the study. Five studies had cohort designs, and the remaining five studies were case reports. The critical appraisal skills program (CASP) checklist for cohort studies was used to assess the risk of bias in the relevant studies[
RESULTS
The databases employed for the search were MEDLINE, EMBASE, COCHRANE, SCOPUS, and DYNAMED, which yielded 22, 46, 20, 3, and 3 papers, respectively. Duplicates in the search, accounting for a total of 26 papers, were removed, leaving a remainder of 68 papers. The titles and abstracts of these 68 papers were read, and 36 irrelevant papers were excluded, along with a conference abstract for which a full text was not available.[
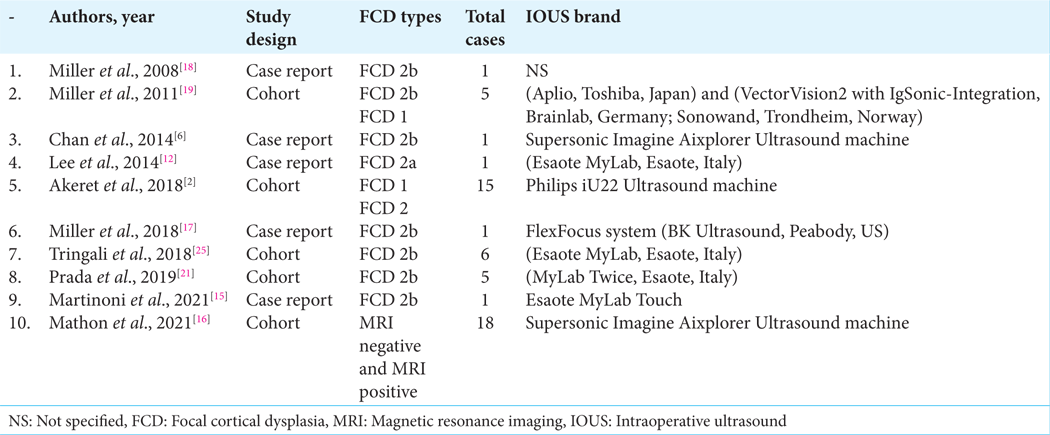
The study with the largest number of patients was by Mathon et al.,[
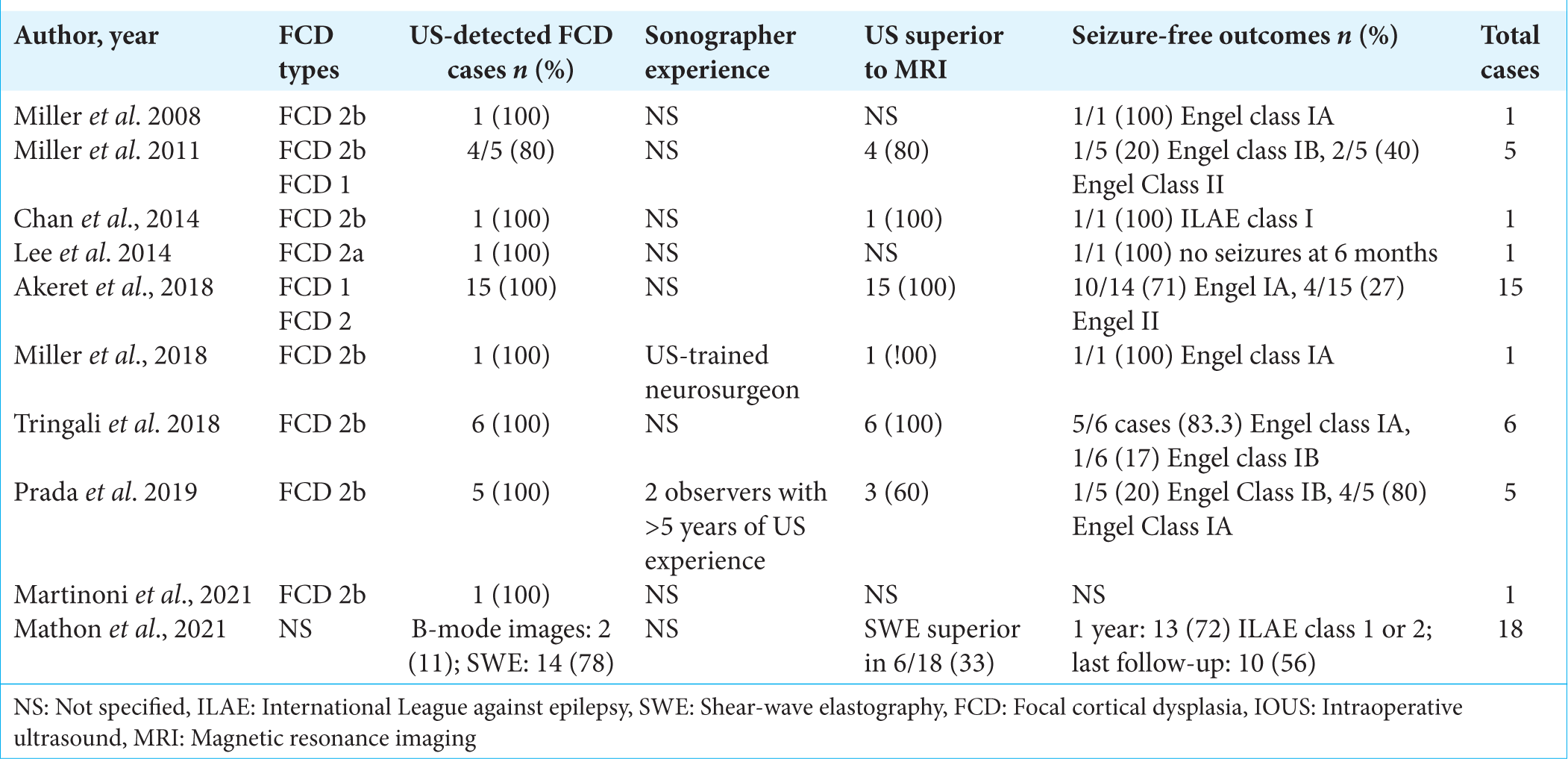
All of the studied papers provided input on ultrasound resolution. The ability of the IOUS to demarcate the FCD boundaries was explained by eight of the ten studied papers. Similarly, eight of the ten papers referred to brain shift correction as a benefit of using IOUS. IOUS successfully detected 78–100% of FCD cases (42 out of a total of 54 patients). Furthermore, the US was superior to MRI in detecting FCD in 33–100% of cases. Postoperative outcomes were mostly explored through the Engel classification of seizure freedom as well as the ILAE classification. Most series reported good outcomes with Engel classes of 1 or 2 or ILAE classes of 1 or 2, reported to range between 56% and 100%. On the other hand, the occurrence of postoperative neurological deficits was mentioned in only six of the papers [
The IOUS transducer brand details were also documented, as was the expertise of the IOUS operator if mentioned by the author. The most commonly used IOUS machine brand was Esaote MyLab, a product from Italy that was used in at least three of the studies. Supersonic Imagine Aixplorer was the second most commonly used IOUS device brand and was used in the two studies that compared IOUS and SWE detection of FCD, as this device is capable of switching between standard B-mode ultrasound and SWE. Only one study did not specify the IOUS brand used.[
Six articles compared traditional ultrasound (B-mode) imaging to other imaging techniques, such as ultrasound using SWE and MRI. Mathon et al. specifically examined the use of SWE in ultrasound imaging and compared it to the standard B-mode images routinely used in ultrasound. SWE allows the viscoelastic tissue characteristics to be analyzed, thereby distinguishing healthy brain tissue from aberrant tissue. It was found that SWE showed better detection of FCD compared to B-mode images, although the findings were not statistically significant (77.8% of cases were detected by SWE, and 11.1% of cases were detected by B-mode images, P = 0.42).[
DISCUSSION
This systematic review identified ten publications that specifically addressed the use of IOUS in FCD surgery, published between the years 2008 and 2022. Publications regarding this specific topic are very scarce. There are no prior reviews that have addressed this topic.
Three factors were examined to measure the surgical success of IOUS-assisted FCD resection surgery: postoperative seizure freedom, neurological deficit, and postoperative MRI findings of residual FCD. Postoperative neurological deficit is reflective of possible injury to an eloquent area of the brain, while the presence of residual FCD on postoperative MRI or incomplete postoperative seizure freedom status is indicative of incomplete FCD resection, which, as mentioned previously, is the most important factor for determining surgical success rates.[
A clear association can be seen between the type of FCD and the surgical success rate. Overall, surgery on FCD type 1 showed a less successful outcome than surgery on FCD type 2 patients. At least two of the papers included FCD type 1 patients.[
The majority of the selected papers commended the use of IOUS in this kind of surgery, particularly for type 2 FCD lesions. Eight studies expressed appreciation of the fact that IOUS corrected brain shift as opposed to the standard neuronavigation system that is built from preoperative MRIs. Specific praise was also shown for the high resolution of the IOUS; in fact, two of the papers declared IOUS’s resolution superior to that of preoperative MRI.[
The foremost limitation of this study is that the topic is rare and has not been studied extensively. Only ten papers met the inclusion criteria of this review; all involved a very small sample size, none were comparative prospective studies, and only two of the papers denied any external funding. Although the results look very promising and show impressive outcomes when using IOUS to assist in FCD resection surgery, it is difficult to draw a firm conclusion considering the limitations mentioned above. The data in the chosen studies additionally showed considerable heterogeneity, thus preventing us from conducting the initially intended meta-analysis. Therefore, we strongly encourage institutions to conduct high-quality comparative, prospective studies that standardize dependent variables such as operator expertise and IOUS brand. Further, research in this direction has the potential to improve the quality of life in many seizure patients.
CONCLUSION
The best treatment modality currently available for drug-refractory patients to improve their quality of life is epilepsy surgery. FCD patients make up 30–50% of all drug-refractory epilepsy cases, and surgery in FCD cases had the least favorable outcome among all other causes of drug-refractory epilepsy. This is a result of the difficulty in distinguishing dysplastic from normal tissue intraoperatively, which is extremely influential because the completeness of resection of the lesion is the most important predictor of the surgical outcome. Unfortunately, studies conducted to seek improvement in intraoperative imaging for this lesion are very sparse. The objective of this paper was to explore the potential of IOUS to improve the outcome of this form of surgery by systematically identifying and critiquing as many relevant papers as possible. This review highlights the potential value of IOUS, especially for ILAE class 2 FCD, which shows potentially better outcomes than those of other modalities such as IoMRI, SWE, and ECoG. Furthermore, this review identified a gap in the literature and highlighted that more high-quality comparative research in this area is warranted, with particular emphasis on ILAE class 1 FCD.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
The authors extend their deepest gratitude to Mr. Niall O’Brien for assisting with the literature search.
References
1. About epilepsy society. Available from: https://epilepsysociety.org.uk/what-we-do/about-epilepsy-society [Last accessed on 2023 Apr 06].
2. Akeret K, Bellut D, Huppertz HJ, Ramantani G, König K, Serra C. Ultrasonographic features of focal cortical dysplasia and their relevance for epilepsy surgery. Neurosurg Focus. 2018. 45: E5
3. Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A. The clinicopathologic spectrum of focal cortical dysplasias: A consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission: The ILAE classification system of FCD. Epilepsia. 2011. 52: 158-74
4. Case R, Blake S, editors. What is epilepsy?. A practical guide to supporting people with epilepsy. Germany: Springer International Publishing; 2020. p. 1-9
5. Casp-uk.net. Available from: https://casp-uk.net/casp-tools-checklists [Last accessed on 2023 Apr 06].
6. Chan HW, Pressler R, Uff C, Gunny R, St Piers K, Cross H. A novel technique of detecting MRI-negative lesion in focal symptomatic epilepsy: Intraoperative ShearWave elastography. Epilepsia. 2014. 55: e30-3
7. Chakraborty A, Uff C, Harkness W. The use of Intraoperative ultrasound and ultrasound elastography for the detection of eleptogenic areas. 39th annual meeting of the international society for pediatric neurosurgery, Goa, India 2011. Childs Nerv Syst. 2011. 27: 1751-850
8. Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD. Long-term outcome after epilepsy surgery for focal cortical dysplasia. J Neurosurg. 2004. 101: 55-65
9. Epilepsy. Available from: https://www.who.int/news-room/fact-sheets/detail/epilepsy [Last accessed on 2023 Apr 06].
10. Jayalakshmi S, Nanda SK, Vooturi S, Vadapalli R, Sudhakar P, Madigubba S. Focal cortical dysplasia and refractory epilepsy: Role of multimodality imaging and outcome of surgery. AJNR Am J Neuroradiol. 2019. 40: 892-8
11. Kabat J, Król P. Ogniskowa dysplazja korowa-stan obecny wiedzy. Pol Przegl Radiol Med Nukl. 2012. 77: 35-43
12. Lee CC, Lin CF, Yu HY, Hung SC, Shih YH, Hsu SP. Applications of intraoperative ultrasound in epilepsy surgery for focal cortical dysplasia. J Med Ultrasound. 2014. 22: 43-6
13. Lee SK, Kim DW. Focal cortical dysplasia and epilepsy surgery. J Epilepsy Res. 2013. 3: 43-7
14. Martinez-Lizana E, Fauser S, Brandt A, Schuler E, Wiegand G, Doostkam S. Long-term seizure outcome in pediatric patients with focal cortical dysplasia undergoing tailored and standard surgical resections. Seizure. 2018. 62: 66-73
15. Martinoni M, Marucci G, Meletti S, Volpi L, Michelucci R, Giulioni M. Ultrasound assisted awake epilepsy surgery for type IIB focal cortical dysplasia in eloquent areas. J Neurosurg Sci. 2021. 65: 75-7
16. Mathon B, Clemenceau S, Carpentier A. Intraoperative ultrasound shear-wave elastography in focal cortical dysplasia surgery. J Clin Med. 2021. 10: 1049
17. Miller D, Carney P, Archer JS, Fitt GJ, Jackson GD, Bulluss KJ. Intraoperative definition of bottom-of-sulcus dysplasia using intraoperative ultrasound and single depth electrode recording-A technical note. J Clin Neurosci. 2018. 48: 191-5
18. Miller D, Knake S, Bauer S, Krakow K, Pagenstecher A, Sure U. Intraoperative ultrasound to define focal cortical dysplasia in epilepsy surgery. Epilepsia. 2008. 49: 156-8
19. Miller D, Knake S, Menzler K, Krakow K, Rosenow F, Sure U. Intraoperative ultrasound in malformations of cortical development. Ultraschall Med. 2011. 32: E69-74
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
21. Prada F, Gennari AG, Del Bene M, Bonoa BC, Quaiaf E, D’Incert L. Intraoperative ultrasonography (ioUS) characteristics of focal cortical dysplasia (FCD) type II b. Seizure. 2019. 69: 80-6
22. Prada F, Gennari AG, Quaia E, D’Incerti L, de Curtis M, DiMeco F. Advanced intraoperative ultrasound (ioUS) techniques in focal cortical dysplasia (FCD) surgery: A preliminary experience on a case series. Clin Neurol Neurosurg. 2020. 198: 106188
23. Saladin KS, editors. Anatomy and physiology: The unity of form and function. United States: McGraw Hill Higher Education; 2011. p.
24. Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971. 34: 369-87
25. Tringali G, Bono B, Dones I, Cordella R, Didato G, Villani F. Multimodal approach for radical excision of focal cortical dysplasia by combining advanced magnetic resonance imaging data to intraoperative ultrasound, electrocorticography, and cortical stimulation: A preliminary experience. World Neurosurg. 2018. 113: e738-46
26. Zhao B, Zhang C, Wang X, Wang Y, Liu C, Mo J. Sulcus-centered resection for focal cortical dysplasia type II: Surgical techniques and outcomes. J Neurosurg. 2020. 135: 266-72