- Department of Neurological Surgery, Renaissance School of Medicine at Stony Brook University, Stony Brook, United States
- Department of Pathology, Renaissance School of Medicine at Stony Brook University, Stony Brook, United States
- Department of Radiology, Renaissance School of Medicine at Stony Brook University, Stony Brook, United States
Correspondence Address:
Reza Dashti, Department of Neurological Surgery, Renaissance School of Medicine at Stony Brook University, Stony Brook, United States.
DOI:10.25259/SNI_969_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jade I. Basem1, Roberta Seidman2, Dinko Franceschi3, Reza Dashti1. Extraneural metastases of glioblastoma: A case report and literature review. 28-Mar-2025;16:102
How to cite this URL: Jade I. Basem1, Roberta Seidman2, Dinko Franceschi3, Reza Dashti1. Extraneural metastases of glioblastoma: A case report and literature review. 28-Mar-2025;16:102. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13463
Abstract
BackgroundGlioblastoma (isocitrate dehydrogenase [IDH]-wildtype, WHO Grade 4) is known to have a high recurrence rate with poor management of morbidity and mortality. Metastatic spread of glioblastomas is rare with extraneural osseous spread having been reported in under 100 cases. In this report, a case of glioblastoma with widespread extraneural metastatic lesions, including distal extremities, is presented.
Case DescriptionA 70-year-old female presented with progressive word-finding difficulty and confusion. Brain magnetic resonance imaging (MRI) revealed a 5 × 7 cm left temporal solid and cystic mass with heterogenous contrast enhancement and significant surrounding edema. She underwent near-total tumor resection, and the pathological diagnosis was glioblastoma, (IDH-wildtype, WHO grade 4), with sarcomatous and primitive neuronal components. She received radiation therapy and temozolomide over 4 months. At 5 months postoperative, she presented with new bilateral lower extremity weakness and left facial droop. MRI and positron emission tomography scans revealed local recurrence and metastatic lesions to vertebrae, extremities, and lymph nodes.
ConclusionPrevious research into rare glioblastoma bone metastases supports the theories of spread through hematogenous routes, surgical disruption, glymphatic system, and potential genetic susceptibility. However, no literature to date can adequately explain the distal limb metastases presented in this case, which shows the necessity for further understanding of this pathology.
Keywords: Case report, Extracranial metastasis, Glioblastoma, Malignant astrocytoma, Osseous metastasis
INTRODUCTION
Malignant astrocytomas are known to be the most common primary central nervous system tumors in adults.[
CASE REPORT
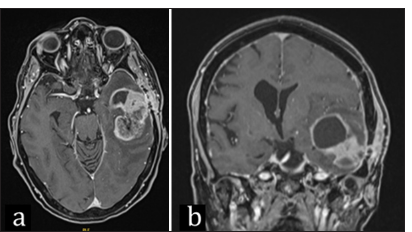
A 70-year-old female with a past medical history of hypertension, hyperlipidemia, and thyroidectomy for medullary thyroid cancer 26 years prior was presented to the emergency room with a 1-week history of word-finding difficulty and confusion. Brain magnetic resonance imaging (MRI) revealed a 5 × 7 cm left temporal mass with solid and cystic components, heterogenous contrast enhancement, and significant surrounding edema [
Figure 2:
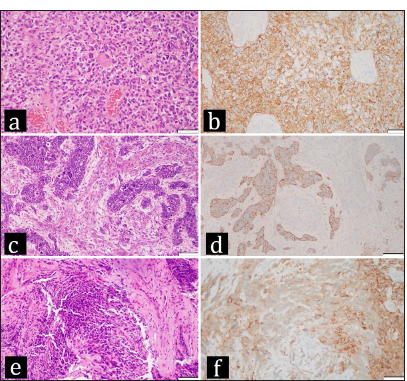
(a-d) Representative areas from the first resection of the brain tumor. (a) Hematoxylin and eosin (H&E) section from a region of the neoplasm that exhibits astrocytic histopathological features. The neoplastic cells, some with subtle fibrillar processes, some with eosinophilic cytoplasm, and some without obvious cytoplasm are haphazardly distributed. Nuclei are pleomorphic, and mitotic activity is present. Multifocal necrosis that is present in the neoplasm is not shown. (b) Most of the cells in the region with glial histomorphology express glial fibrillary acidic protein (GFAP), identified by the brown staining, as seen in this GFAP immunohistochemical preparation. (c) H&E section from a region of the brain tumor that consists of circumscribed nests of primitive neuronal cells with hyperchromatic blue nuclei and little visible cytoplasm distributed among spindle cells that represent a sarcomatous component within the neoplasm. (d) Synaptophysin immunohistochemistry demonstrates synaptophysin expression in the cells that are arranged in nests that represent a primitive neuronal component; the surrounding unstained cells comprise the sarcomatous component in the neoplasm. (e and f) show a representative area from the thoracic vertebral lesion, which extended into the epidural space. (e) The H&E section demonstrates abundant cells with hyperchromatic nuclei and little cytoplasm, characteristic of small blue cells. (f) Synaptophysin immunohistochemistry from the area shown in e demonstrates that these small blue cells express synaptophysin, consistent with their origin from the primitive neuronal component of the brain tumor. (a, b, e, f) - 200x magnification, scale bar = 50 microns; (c and d) - 100x magnification scale bar = 100 microns.
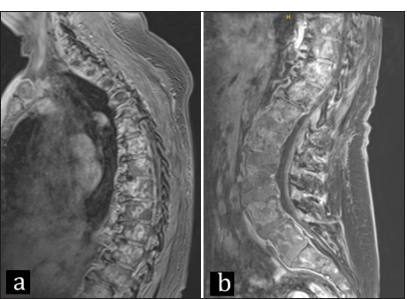
Local recurrence measuring as 5 × 5 cm cystic mass was detected on a 5-month imaging follow-up, and surgical resection was planned [
Figure 4:
7-month positron emission tomography scan with metastases seen in the tibia, humerus, left supraclavicular lymph node, mediastinal lymph nodes, suboccipital lymph nodes, skull, right temporal bone, clivus, entire spine, ribs, sternum, and pelvis. (a) Axial, (b) sagittal, (c and d) anterior view.
DISCUSSION
The case presented here represents a left temporal glioblastoma (IDH-wildtype, WHO grade 4) with a distribution of metastatic disease that has not been previously reported, both in the combination of metastases seen in the bone, soft tissue, and lymph nodes together with the spread to the distal extremities. There are several theories regarding glioblastoma spread to bone based on the limited case presentations in the literature, with many agreeing that comprehension of this pathology is limited due to the short survival time of these patients.
Strong et al. report many similarities between our case and previous literature, such that 24% of bone metastases occur in the thoracic region, 36% of patients receive the triad of chemotherapy, surgery, and radiotherapy, and thrombocytopenia is a common late-stage finding.[
Extensive metastatic lesions to vertebral and distal extremity bones can be explained by theories suggesting spread is surgical disruption of the original tumor area, although many studies have shown that this is not a prerequisite for metastasis.[
In addition to the osseous spread, there were also metastases found in the lymph node, cervical soft tissue, and lungs. The newly reported glymphatic system has been speculated to play a role in the spread to these areas, such as through a recent MR imaging study by Albayram et al. (2022) that proved the ventral meningeal lymphatics system with a direct route between cranial nerves and cervical lymph nodes.[
One of the most compelling current theories for soft tissue and organ spread is the ability of glioblastomas to avoid immune surveillance through the presence of circulating tumor cells (CTCs) in 20–39% of patients and case reports of organ donor recipients that later developed glioblastomas.[
There are several limitations to understanding this pathology, with a significant one being the short survival time limiting the metastases that are able to present in these cases before death. It makes understanding this spread incredibly difficult and each case an important study. It has also been postulated that this may be the reason for the lack of distal metastases seen in the literature before our reported case.[
CONCLUSION
Previous research into rare glioblastoma bone metastases supports the theories of spread through hematogenous routes, surgical disruption, glymphatic system, and potential genetic susceptibility. However, no literature to date can adequately explain the distal limb metastases presented in this case, which shows the necessity for further understanding of this pathology.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We want to acknowledge all members of the Dashti Lab for their continued support.
References
1. Albayram MS, Smith G, Tufan F, Tuna IS, Bostancıklıoğlu M, Zile M. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022. 13: 203
2. Alhoulaiby S, Abdulrahman A, Alouni G, Mahfoud M, Shihabi Z. Extra-CNS metastasis of glioblastoma multiforme to cervical lymph nodes and parotid gland: A case report. Clin Case Rep. 2020. 8: 1672-7
3. Armstrong TS, Prabhu S, Aldape K, Hossan B, Kang S, Childress A. A case of soft tissue metastasis from glioblastoma and review of the literature. J Neurooncol. 2011. 103: 167-72
4. Chen H, Shah AS, Girgis RE, Grossman SA. Transmission of glioblastoma multiforme after bilateral lung transplantation. J Clin Oncol. 2008. 26: 3284-5
5. Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018. 24: 1459-68
6. Conte B, Rich BJ, Gultekin SH, Azzam G, Del Pilar Guillermo Prieto Eibl M. A Case of glioblastoma, isocitrate dehydrogenase wild type, with widely disseminated osseous metastasis. Cureus. 2022. 14: e28803
7. Costa RB, Costa R, Kaplan J, Cruz MR, Shah H, Matsangou M. A Rare case of glioblastoma multiforme with osseous metastases. Case Rep Oncol Med. 2017. 2017: 2938319
8. Fabi A, Vidiri A, Carapella C, Pace A, Occhipinti E, Caroli F. Bone metastasis from glioblastoma multiforme without central nervous system relapse: A case report. Anticancer Res. 2004. 24: 2563-5
9. Franceschi S, Lessi F, Aretini P, Mazzanti CM, Menicagli M, La Ferla M. Molecular portrait of a rare case of metastatic glioblastoma: Somatic and germline mutations using whole-exome sequencing. Neuro Oncol. 2016. 18: 298-300
10. Ghous G, Miller D, Doll D, Tuncer T. A Rare case of glioblastoma with extensive liver metastases. Oncology (Williston Park). 2021. 35: 733-40
11. Hamilton JD, Rapp M, Schneiderhan T, Sabel M, Hayman A, Scherer A. Glioblastoma multiforme metastasis outside the CNS: Three case reports and possible mechanisms of escape. J Clin Oncol. 2014. 32: e80-4
12. Hira VV, Wormer JR, Kakar H, Breznik B, van der Swaan B, Hulsbos R. Periarteriolar glioblastoma stem cell niches express bone marrow hematopoietic stem cell niche proteins. J Histochem Cytochem. 2018. 66: 155-73
13. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012. 4: 147ra111
14. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics 2007 CA Cancer. J Clin. 2007. 57: 43-66
15. Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic system: A beginner’s guide. Neurochem Res. 2015. 40: 2583-99
16. Jimsheleishvili S, Alshareef AT, Papadimitriou K, Bregy A, Shah AH, Graham RM. Extracranial glioblastoma in transplant recipients. J Cancer Res Clin Oncol. 2014. 140: 801-7
17. Johansen MD, Rochat P, Law I, Scheie D, Poulsen HS, Muhic A. Presentation of two cases with early extracranial metastases from glioblastoma and review of the literature. Case Rep Oncol Med. 2016. 2016: 8190950
18. Lin ZX, Yang LJ, Huang Q, Fu J. Activated vascular endothelia regulate invasion of glioma cells through expression of fibronectin. Chin Med J (Engl). 2010. 123: 1754-61
19. Liu C, Wang X, Genchev GZ, Lu H. Multi-omics facilitated variable selection in Cox-regression model for cancer prognosis prediction. Methods. 2017. 124: 100-7
20. Liu J, Shen L, Tang G, Tang S, Kuang W, Li H. Multiple extracranial metastases from glioblastoma multiforme: A case report and literature review. J Int Med Res. 2020. 48: 300060520930459
21. Liwnicz BH, Rubinstein LJ. The pathways of extraneural spread in metastasizing gliomas: A report of three cases and critical review of the literature. Hum Pathol. 1979. 10: 453-67
22. Mentrikoski M, Johnson MD, Korones DN, Scott GA. Glioblastoma multiforme in skin: A report of 2 cases and review of the literature. Am J Dermatopathol. 2008. 30: 381-4
23. Mohme M, Maire CL, Schliffke S, Joosse SA, Alawi M, Matschke J. Molecular profiling of an osseous metastasis in glioblastoma during checkpoint inhibition: Potential mechanisms of immune escape. Acta Neuropathol Commun. 2020. 8: 28
24. Müller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014. 6: 247ra101
25. Noch EK, Sait SF, Farooq S, Trippett TM, Miller AM. A case series of extraneural metastatic glioblastoma at Memorial Sloan Kettering Cancer Center. Neurooncol Pract. 2021. 8: 325-36
26. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021. 23: iii1-105
27. Park CC, Hartmann C, Folkerth R, Loeffler JS, Wen PY, Fine HA. Systemic metastasis in glioblastoma may represent the emergence of neoplastic subclones. J Neuropathol Exp Neurol. 2000. 59: 1044-50
28. Pasquier B, Pasquier D, N’Golet A, Panh MH, Couderc P. Extraneural metastases of astrocytomas and glioblastomas: Clinicopathological study of two cases and review of literature. Cancer. 1980. 45: 112-25
29. Ray A, Manjila S, Hdeib AM, Radhakrishnan A, Nock CJ, Cohen ML. Extracranial metastasis of gliobastoma: Three illustrative cases and current review of the molecular pathology and management strategies. Mol Clin Oncol. 2015. 3: 479-86
30. Sharma P, Aaroe A, Liang J, Puduvalli VK. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol Adv. 2023. 5: vdad009
31. Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg. 1969. 31: 50-8
32. Stoyanov GS, Petkova L, Iliev B, Ali M, Toncheva B, Georgiev R. Extracranial glioblastoma metastasis: A neuropathological case report. Cureus. 2023. 15: e35803
33. Strong MJ, Koduri S, Allison JA, Pesavento CM, Ogunsola S, Ogunsola O. Bone metastasis from glioblastoma: A systematic review. J Neurooncol. 2022. 158: 379-92
34. Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014. 23: 1985-96
35. Val-Bernal F, Ruiz JC, Cotorruelo JG, Arias M. Glioblastoma multiforme of donor origin after renal transplantation: Report of a case. Hum Pathol. 1993. 24: 1256-9
36. Zapata Laguado M, Baez JM, Luna A, Mantilla C, Palencia M. Bone metastasis from glioblastoma multiforme: A case report. Cureus. 2022. 14: e25464
37. Zhang W, Cai YY, Wang XL, Wang XX, Li Y, Han GY. Bone metastases of glioblastoma: A case report and review of the literature. Front Oncol. 2021. 11: 705455
38. Zhen L, Yufeng C, Zhenyu S, Lei X. Multiple extracranial metastases from secondary glioblastoma multiforme: A case report and review of the literature. J Neurooncol. 2010. 97: 451-7