- Department of Neurosurgery, Services Institute of Medical Sciences, Lahore, Pakistan
- Department of Neurosurgery, Allama Iqbal Medical College, Jinnah Hospital, Lahore, Pakistan
- School of Medicine, Allama Iqbal Medical College, Lahore, Pakistan,
- Wolfson School of Medicine, University of Glasgow, Scotland, United Kingdom
- Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom,
- Department of Neurosurgery, King Edward Medical University, Mayo Hospital, Lahore, Pakistan
- University of Health Sciences, Lahore, Pakistan.
Correspondence Address:
Fauzia Sajjad, Department of Neurosurgery, Allama Iqbal Medical College, Jinnah Hospital, Lahore, Pakistan.
DOI:10.25259/SNI_581_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Fauzia Sajjad1,2, Maryam Farhan Baloch3, Mohammad Ashraf4, Conor S. Gillespie5, Hira Umar1, Ammara Zafar1, Javaria Zulfiqar1, Imdad Ullah1, Sundus Ali6, Naveed Ashraf7. Focal dystonia and ataxic hemiparesis as the initial presentation of a thalamic tuberculoma: A diagnostic challenge in an immunocompetent pediatric patient. 29-Sep-2023;14:350
How to cite this URL: Fauzia Sajjad1,2, Maryam Farhan Baloch3, Mohammad Ashraf4, Conor S. Gillespie5, Hira Umar1, Ammara Zafar1, Javaria Zulfiqar1, Imdad Ullah1, Sundus Ali6, Naveed Ashraf7. Focal dystonia and ataxic hemiparesis as the initial presentation of a thalamic tuberculoma: A diagnostic challenge in an immunocompetent pediatric patient. 29-Sep-2023;14:350. Available from: https://surgicalneurologyint.com/surgicalint-articles/12570/
Abstract
Background: Central nervous system (CNS) tuberculomas are rare and account for approximately 1% of all tuberculosis (TB) cases. These intracranial lesions are more commonly observed in immunocompromised individuals, often as part of disseminated miliary TB or after latent infection reactivation. This case report presents the occurrence of a thalamic tuberculoma in an immunocompetent girl.
Case Description: An 11-year-old girl presented with a 3-month history of progressive right-sided ataxic hemiparesis, hand dystonia/thalamic hand, and headache. There was only a mildly elevated erythrocyte sedimentation rate (25 mm/h.), and her remaining biochemistry and vitals were unremarkable. Magnetic resonance imaging (MRI) brain revealed an ill-defined intra-axial heterogeneous lobulated lesion with crenated margins involving the thalamus and the posterior limb of the internal capsule with significant vasogenic edema. Given the clinical picture, the working diagnosis was a high-grade brain tumor. Due to the absence of a viable operative corridor for a meaningful resection and the diagnostic uncertainty, a stereotactic biopsy was performed, and histopathological analysis confirmed the presence of granulomas consistent with TB. A human immunodeficiency virus test (negative) and interferon-gamma release assay (positive) were then obtained. The patient was commenced on a regimen of anti-TB drugs with a tapering steroid dose. At 8 months, her most recent MRI showed a significant reduction in the size of her tuberculoma, and there is a complete resolution of her hand dystonia and hemiparesis to allow for independence in her activities of daily living.
Conclusion: This report emphasizes the importance of considering causes other than degenerative, vascular, or neoplasms in patients with hemiparesis with dystonia. CNS tuberculomas can present as such without prior history or specific clinical symptoms of TB, making them a diagnostic challenge. In cases with such uncertainty regarding the nature of an intracranial lesion and the role of resection, a stereotactic biopsy is invaluable.
Keywords: Ataxic hemiparesis, Immune competent, Intracranial tuberculoma, Lacunary syndrome, Thalamic hand, Thalamus
INTRODUCTION
Neurological deficits from ischemia, infarction, and mass lesions are common presentations in neurology/ neurosurgery. Lacunar syndrome is a neurological deficit from a deep brain lesion, most commonly ischemia/ infarctions.[
CASE DESCRIPTION
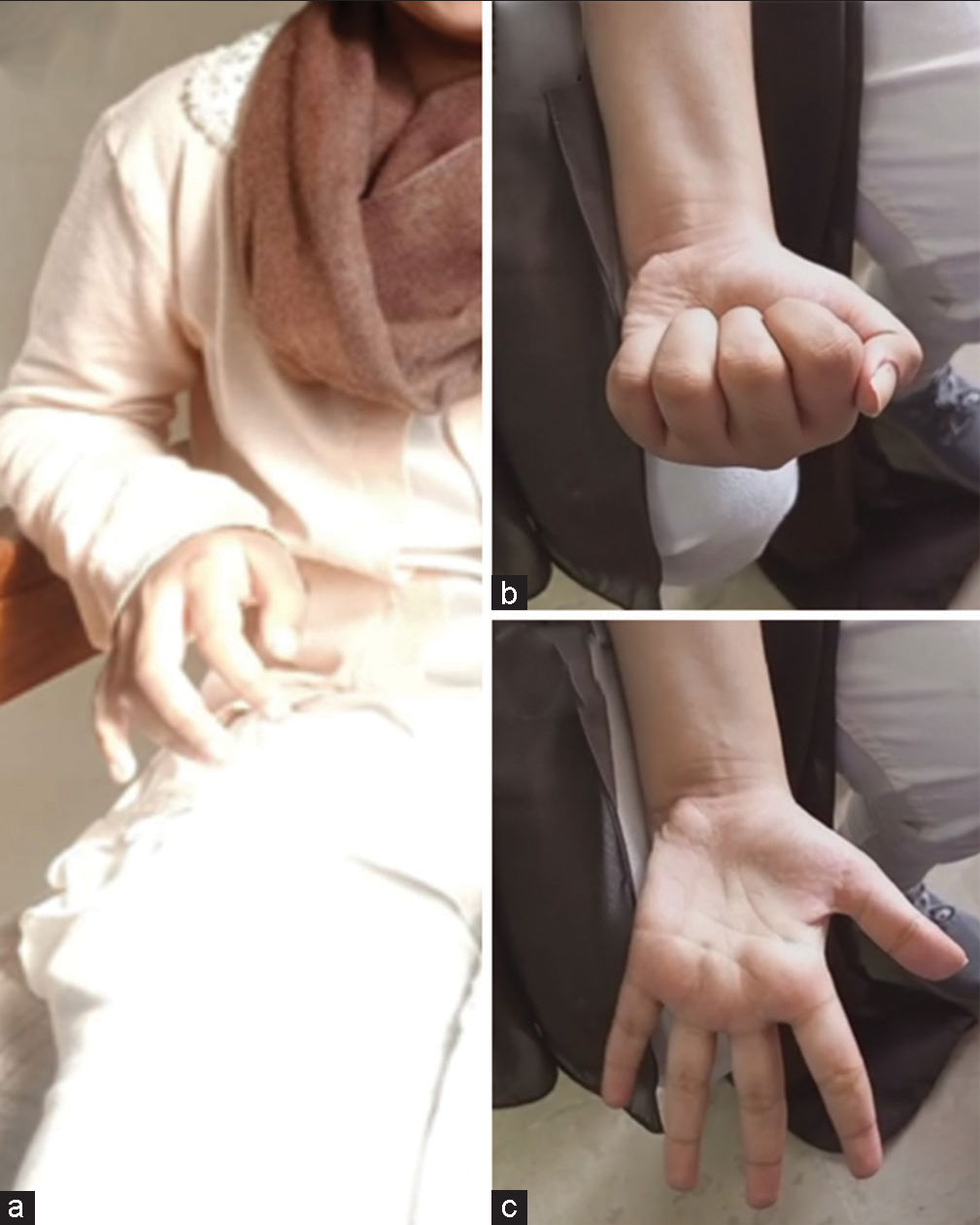
An 11-year-old female presented to our outpatient department complaining of right-sided weakness and headache, which had progressively worsened over 2–3 months. A thorough clinical examination revealed power 4/5 (Medical Research Council scale) in her right limbs with an upgoing plantar. The patient had an ataxic gait and focal dystonia in the form of a “thalamic hand” [
Figure 1:
Left column (a) showing the patient’s preoperative dystonic, “thalamic,” hand that was contracted in this position indefinitely from November 2022. The right column (b and c) from June 2023 illustrates the complete resolution of the dystonia and a full range of movements and power of the initially hemiparetic side.
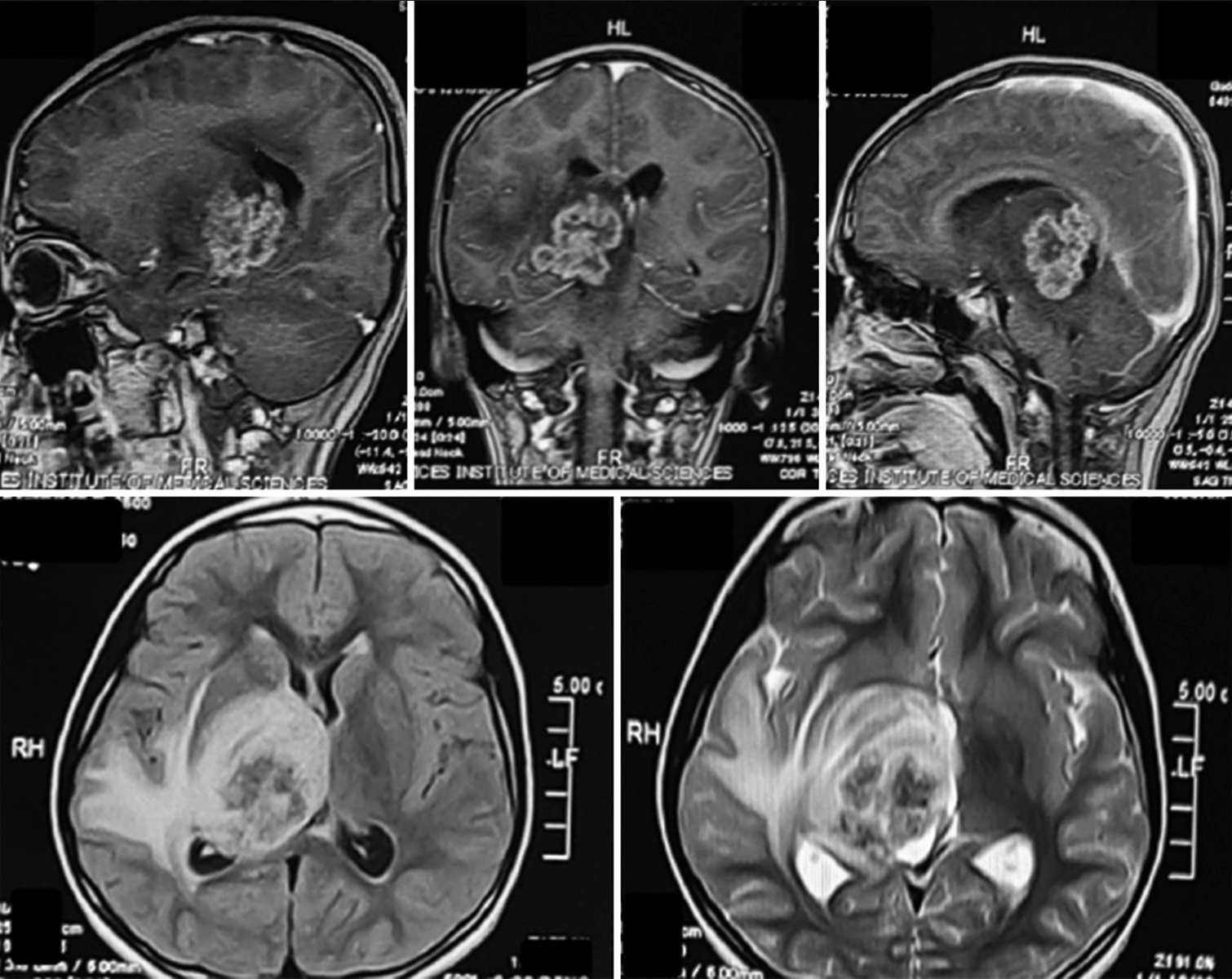
Figure 2:
Top row T1 contrast; (bottom left) Fluid attenuated inversion recovery (FLAIR) sequence; (bottom right) T2 sequence. From radiology report – An ill-defined intra-axial heterogeneous lobulated lesion with crenated margins demonstrating patchy contrast enhancement, returning T2 and FLAIR signals involving the right thalamus, posterior limb of the internal capsule, the right half of the midbrain, ipsilateral temporal lobe, hippocampal region, and abutting the splenium of the corpus callosum. There is marked perilesional vasogenic edema. The lesion exerts a mass effect on the body and trigone of the ipsilateral and third ventricles.
Considering the patient’s clinical history and young age, the radiologist reported the differentials as a supratentorial primitive neuroectodermal tumor, extraventricular neurocytoma, or any other high-grade glioma. We, initially, planned to excise the lesion, but given the deep-seated nature of the lesion and the lack of a viable operative corridor, it was decided to perform a stereotactic biopsy as the neurosurgical working differential was of a high-grade tumor; we did not want to risk neurological deficits in the face of our provisional diagnosis and planned to utilize chemo/radiotherapy as/if indicated. A stereotactic biopsy was performed on the 4th day using an inomed frame. Biopsy microspecimens revealed caseous necrosis and necrotizing granulomatous inflammation. Histologically, scattered granulomas comprised epithelioid histiocytes with admixed lymphocytes, plasma cells, and giant cells were observed. Immunohistochemical staining was positive for CD3 (indicating reactivated T-cells), CD20 (indicating reactivated B cells), negative for Grocott methenamine silver stain (fungus), and negative for Ziehl-Neelsen stain. As the patient belonged to an impoverished socioeconomic background, TB was now considered; however, there was no history of constitutional/reactivation of TB symptoms (fever, night sweats, weight loss, and hemoptysis) or family history of TB, which we now specifically enquired regarding. The patient also received a complete course of the BCG vaccination during infancy as part of the national immunization program. No biochemical or clinical evidence of reactivated, milliary, or CNS TB was found except for the raised ESR, which was now in conformity with the histopathology. An interferon-gamma release assay (positive) and human immunodeficiency virus test (negative) were obtained.
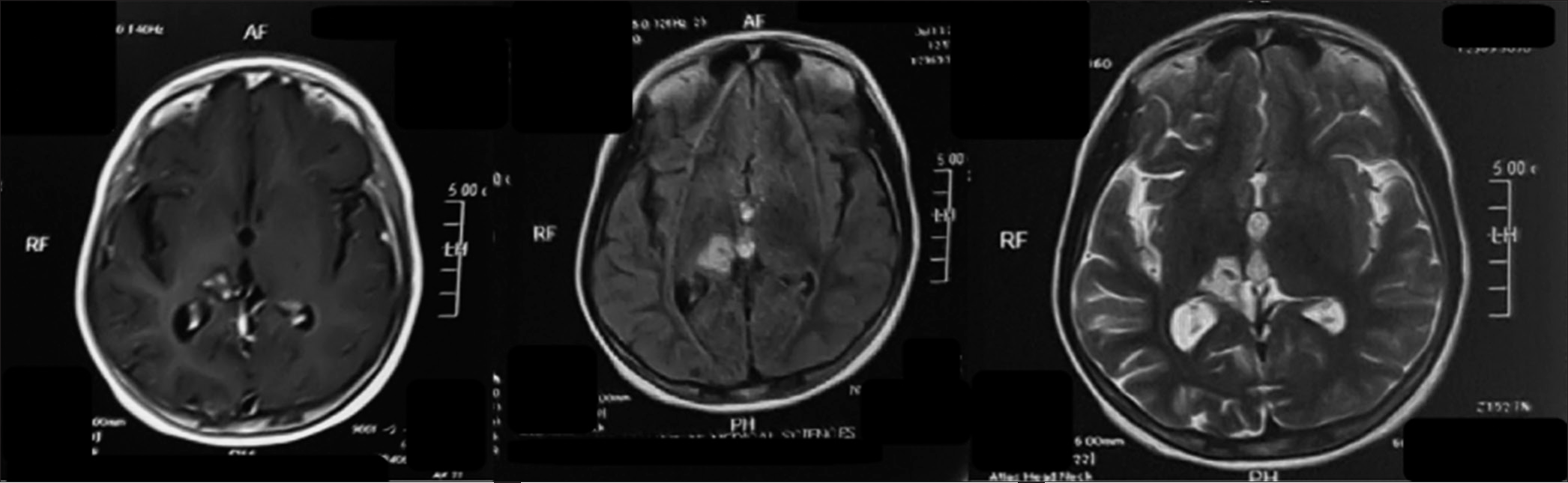
The patient was eventually discharged on the 14th post admission day, with the family counseled diligently on the importance of compliance with the prescribed 18-month anti-TB drugs. The patient was commenced on a steroid tapering regimen with isoniazid, rifampicin, pyrazinamide, and ethambutol for the first 2 months and is now on isoniazid and rifampicin. At her 8th-month follow-up, her dystonic hand has resolved to complete normality, and she has regained a complete range of motion in her right limbs [
DISCUSSION
TB is a significant global public health concern, especially in underdeveloped nations.[
CNS tuberculomas are a rare condition that accounts for approximately 1% of all TB.[
According to the British Infection Society Guidelines, children with CNS TB should ideally be managed by a doctor conversant with and knowledgeable about pediatric TB or by a pediatric infectious diseases unit.[
CONCLUSION
Without a clinical history or specific symptom, a CNS tuberculoma’s clinical and radiological presentation can be non-specific and may indicate a wide range of differentials. A cautious approach in such cases is advised to affirm a diagnosis before definitive treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alarcón F, Tolosa E, Muñoz E. Focal limb dystonia in a patient with a cerebellar mass. Arch Neurol. 2001. 58: 1125-7
2. Das JM, Sugatha Rao SC, Bhanu Prabhakar R. The successful surgical management of a rare case of infratentorial tubercular abscess in an immunocompetent pediatric patient. Indian J Tuberc. 2021. 68: 529-33
3. Datta AK, Chakraborty U, Chandra A. The thalamic hand: An enigmatic sequela of thalamic stroke. QJM. 2022. 115: 239-40
4. Davis LE, Scheld WM, Whitley RJ, Durack DT, editors. Infections of the central nervous system. New York: Elsevier, Raven Press; 1991, 1993. p. 937
5. Ehrt S, Schnappinger D, Rhee KY. Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat Rev Microbiol. 2018. 16: 496-507
6. Fisher CM. Capsular infarcts: The underlying vascular lesions. Arch Neurol. 1979. 36: 65-73
7. Katelaris AL, Jackson C, Southern J, Gupta RK, Drobniewski F, Lalvani A. Effectiveness of BCG vaccination against Mycobacterium tuberculosis infection in adults: A cross-sectional analysis of a UK-based cohort. J Infect Dis. 2020. 221: 146-55
8. Mashabela GT, de Wet TJ, Warner DF. Mycobacterium tuberculosis metabolism. Microbiol Spectr. 2019. 7: a021121
9. Mohamud JA, Gu J. Aggressive intracerebral tuberculoma in a 1-year-old child: A case report with literature review. Radiol Case Rep. 2020. 15: 2282-4
10. Sahu R, Patil TB, Kori P, Shukla R. Isolated thalamic tuberculoma presenting as ataxic hemiparesis. BMJ Case Rep. 2013. 2013: bcr2013009100
11. Seddon JA, Shingadia D. Epidemiology and disease burden of tuberculosis in children: A global perspective. Infect Drug Resist. 2014. 7: 153-65
12. Soto-Cabrera E, Villamil-Osorio LV, Garcia-Luna RC, CarreraPineda R. Optochiasmatic tuberculomas as a paradoxical reaction to treatment for meningeal tuberculosis. Rev Neurol. 2018. 66: 286-8
13. Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009. 59: 167-87
14. World Health Organizati, editors. Staff WHO. Global tuberculosis report 2013. Geneva: World Health Organization; 2013. p.