- Departments of Neurosurgery, Centro Diagnostico Docente Las Mercedes, Hospital de Clinicas Caracas, Caracas, Venezuela,
- Departments of Radiation Oncology, Centro Diagnostico Docente Las Mercedes, Hospital de Clinicas Caracas, Caracas, Venezuela,
- Department of Medicine, School of Medicine and Public Health, University of Wisconsin, Madison, United States.
Correspondence Address:
Salvador Somaza
Department of Medicine, School of Medicine and Public Health, University of Wisconsin, Madison, United States.
DOI:10.25259/SNI-134-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Salvador Somaza, Eglee M. Montilla, Maria C. Mora. Gamma knife radiosurgery on the trigeminal ganglion for idiopathic trigeminal neuralgia: Results and review of the literature. 07-Jun-2019;10:89
How to cite this URL: Salvador Somaza, Eglee M. Montilla, Maria C. Mora. Gamma knife radiosurgery on the trigeminal ganglion for idiopathic trigeminal neuralgia: Results and review of the literature. 07-Jun-2019;10:89. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9359
Abstract
Background: In the present study, we evaluate the results of gamma knife surgery (GKS) for the treatment of trigeminal neuralgia (TN) using the trigeminal ganglion (TG’) and the adjacent fibers of trigeminal nerve as a target.
Methods: From February 2013 to July 2017, we treated 30 cases of TN with GKS. In this group, all patients had an idiopathic typical TN. The radiosurgical target was conformed through two isocenters, 8 and 4 mm at the cavum de Meckel. The maximum dose was 86 Gy using the isodose line of 50%. The median age of the patients was 58.5 (range 28–94) years old, and the median time from diagnosis to GKS was 94 months (range 13–480 months). The median follow-up was 28.5 (range 12–49) months. Clinical outcomes were analyzed. Univariate and multivariate analyses were performed to evaluate factors that correlated with a favorable, pain-free outcome.
Results: The mean time to relief of pain was 7 (range 1–40) days. The percentage of patients with significant pain relief was 93.3%. Relapse in pain was noted in four patients at 3, 16, 19, and 36 months. Nine patients were treated in acute status. Fourteen patients had intense pain between 1 and 7 days before the procedure. Among those with the recurrence of their symptoms, one patient had a microvascular decompression. Multivariate regression adjusted for age and sex suggests that, by 40 months, 70% of the patients treated with radiosurgery will remain pain free. At the last follow-up, GKS resulted in pain relief in 86.6% of patients. Our analysis suggests that, using this technique, we can expect that approximately 70% of patients with TN will have some degree of pain improvement at 3 years’ post radiosurgery.
Conclusions: GKS on TG appears to be a reasonable treatment option with short latency period, minor collateral effects, and high percentage of pain control. The mechanism of action of radiosurgery could be related to the inactivation of the satellite glial cells in the TG.
Keywords: Facial pain, Gamma knife, Gasser ganglion, Radiosurgery, Satellite glial cells, Trigeminal ganglion, Trigeminal neuralgia
INTRODUCTION
Trigeminal neuralgia (TN) is clinically characterized by severe paroxysmal facial pain and brief, sharp, or stabbing unilateral electric shock-like sensations often triggered by facial movements or cutaneous stimulation.[
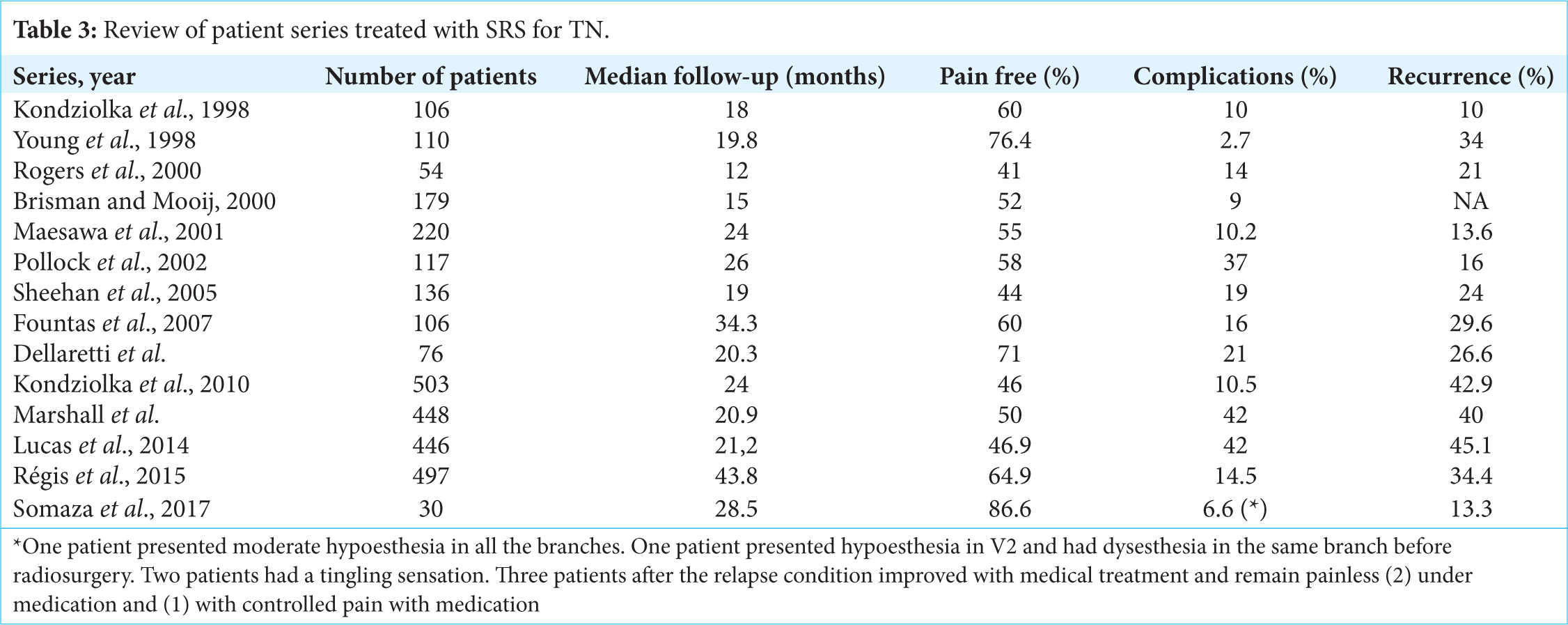
Although many reports have documented the efficacy of radiosurgery in the treatment of TN, controversy remains regarding the optimal treatment dose, target site, and brainstem dose.[
In 1971, Leksell[
In 2014, we reported[
Due to the excellent results obtained, we decided to prospectively evaluate the technique in 30 patients with idiopathic TN. The results are presented and discussed.
METHODS
Patient population
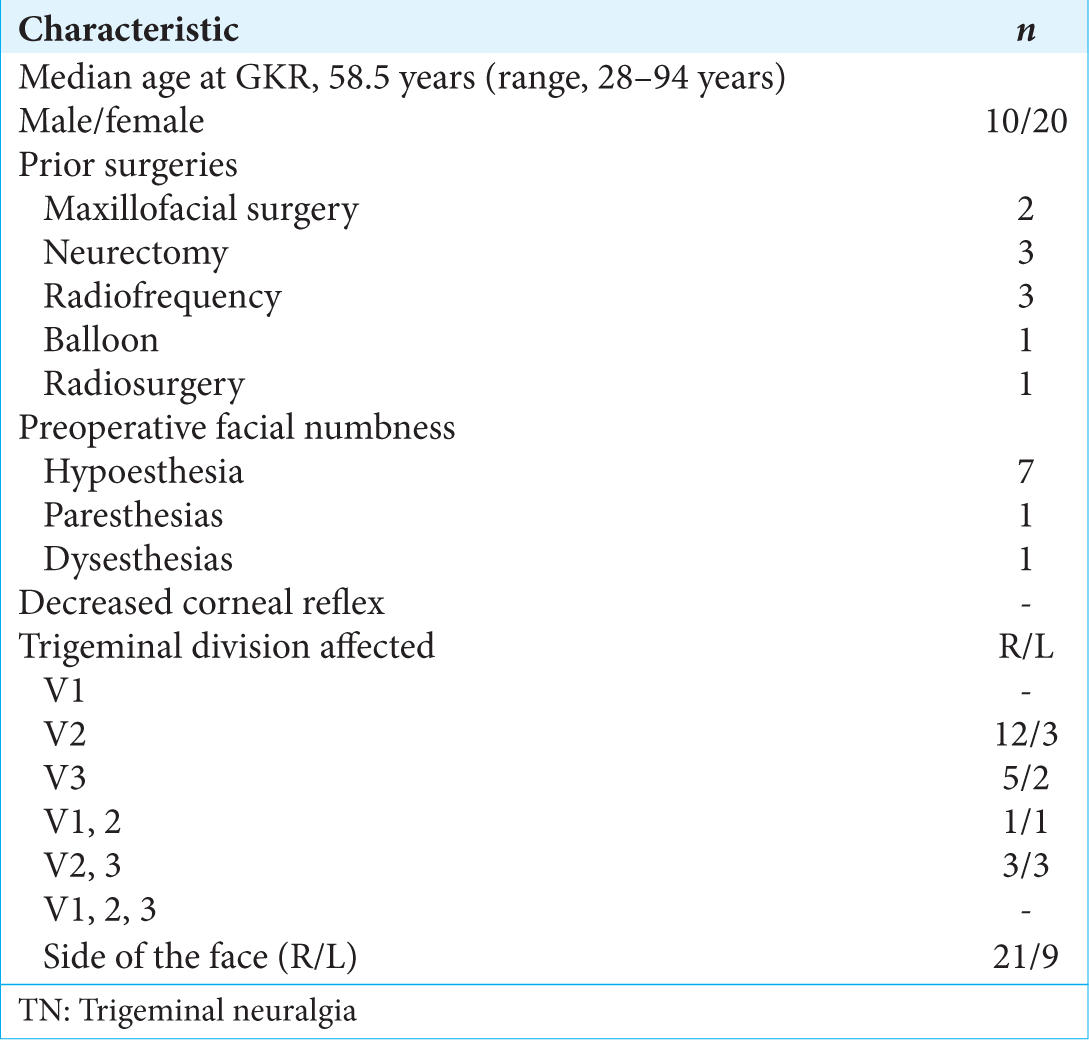
Between February 2013 and July 2017, a prospective study was conducted to analyze the responses of 30 consecutive patients with typical TN refractory to therapy that treated with gamma knife surgery (GKS) at the gamma knife center las mercedes (CDD) in Caracas, Venezuela. The median age of the patients was 58.5 years (range, 28–94 years) and the mean duration pain was 94 months (range, 13–480 months.). In the distribution of pain, a combination of branches was more frequently involved in all patients. The right side of the face was more frequently involved (71.8%). Numbness was noted in nine patients (28.1%), eight of which had previous local or invasive therapeutic procedures.
All patients received pharmacotherapy which included gabapentin, pregabalin, carbamazepine, and combinations. Ten patients had a history of previous surgical intervention, including local blocks of trigeminal branches (n = 8), cryotherapy (n = 2), radiofrequency (n = 4), peripheral neurectomy (n = 3), maxillofacial surgeries (n = 5), balloon compression (n = 1), and previous radiosurgery (n = 1). No patients had anesthesia Dolorosa. Clinical characteristics are shown in
Associated pathologies were arterial hypertension (n = 17), obesity (n = 2), diabetes mellitus (n = 1), hypothyroidism (n = 1), and hemochromatosis (n = 1).
As part of our protocol for TN radiosurgery, all patients underwent clinical examination and neuroimaging with computed tomography (CT) or magnetic resonance imaging (MRI) to exclude other pathologies that could produce TN (tumors, compression by the complex of vertebrobasilar arteries and other vascular malformations, and multiple sclerosis). All patients fulfilled the criteria of the International Headache Society and had a TN1.
Criteria for using GKS
The decision to use GKS was based on criteria such as the medical condition of the patients, pain refractory to pharmacologic treatment, significant adverse effects from medication, previous unsuccessful surgical procedures, the absence of vascular or tumor compression of the involved trigeminal nerve on the MR images, and the patients’ willingness to undergo radiosurgery instead of other techniques.
Radiosurgical technique
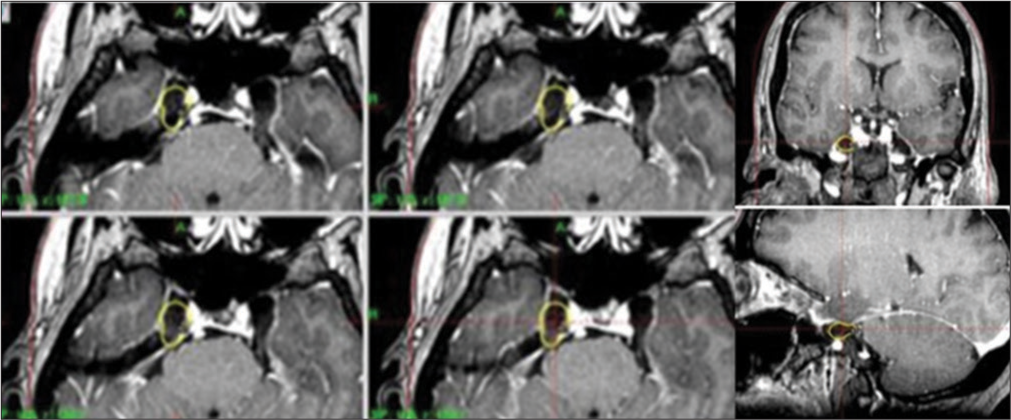
All patients underwent SRS using a gamma knife (Leksell Gamma Knife, Elekta Instruments, Atlanta, Ga), model C. Under mild sedation and local anesthesia, the Leksell Model G stereotactic frame (Elekta Instruments) was applied. Stereotactic MRI was performed on each patient to identify the trigeminal nerve and its course from the pons, using contrast enhancement. A three-dimensional time-of-flight (3D-TOF) sequence was performed using a 1.5-Tesla MRI scanner (General Electric Signa HD). Fast imaging, employing steady-state acquisition sequence, provides images included in the MR protocol. A treatment plan was implemented using the Leksell Gamma Plan treatment planning system (Elekta AB).
Stereotactic coordinates were calculated for two isocenters; one 8-mm isocenter was placed on the Meckel’s Cavum and a 4 mm was placed at the adjacent fibers of the trigeminal nerve from the TG. The maximum dose was 86 Gy (43 Gy prescribed to the 50% isodose) [
Patient outcomes and follow-up evaluation
All patients were discharged the same day after radiosurgery. They were instructed to continue their preoperative medications after the radiosurgical procedure and to gradually taper their medications over 16-week intervals.
The primary outcome after radiosurgery was based on pain intensity which was defined and assessed using the barrow neurological institute (BNI) pain intensity scoring criteria. The mean follow-up time after radiosurgery was 28.5 months (range, 12–49 months). An assessment of outcomes was performed by a blinded evaluator, trained to complete telephone follow-ups and clinical examinations as part of our protocol. It was then followed by a clinical consultation in our center, every 4 months; all of which allowed to keep updated patients’ records. Clinical evaluation included facial sensory testing, corneal reflex, and jaw motility. We evaluated the degree of pain relief, latency interval to pain relief, drugs used, development of new signs or symptoms, and the need for and response to additional surgical procedures.
Statistical analysis
Categorical variables were compared through Chi-square testing. For continuous variables, Student’s t-test was used for normally distributed variables (e.g., age); Kruskal–Wallis tests were used for non normally distributed variables (e.g., length of stay). P < 0.05 was considered to be statistically significant. Cox proportional regression was used to generate a predicted pain-free survival curve, adjusting for age and sex of patients. All statistical analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Clinical response
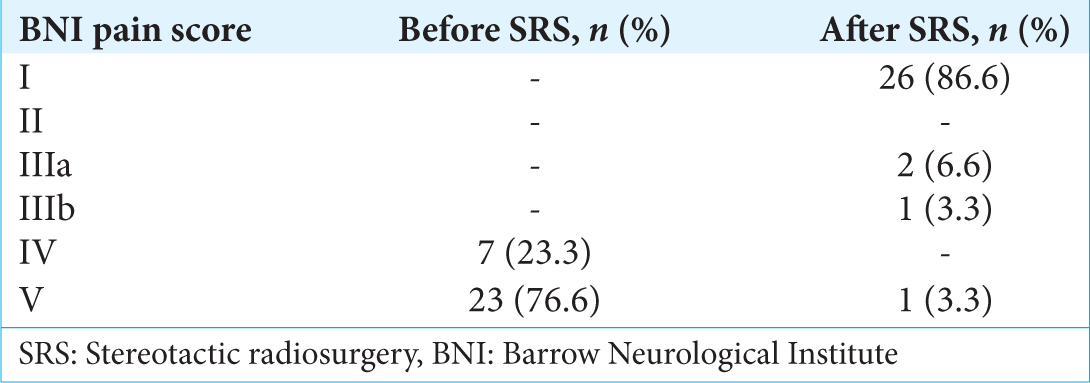
Before undergoing GKS, all patients categorized their pain as BNI IV or V. The BNI pain score at baseline and last follow-up is shown in
The mean latency period was 7 days (range, 1–40 days); all patients were pain free. The initial response rate in the patients was 100%, including patients with previous local and ablative procedures. In 18 patients (60%), pain relief was obtained before 8 days.
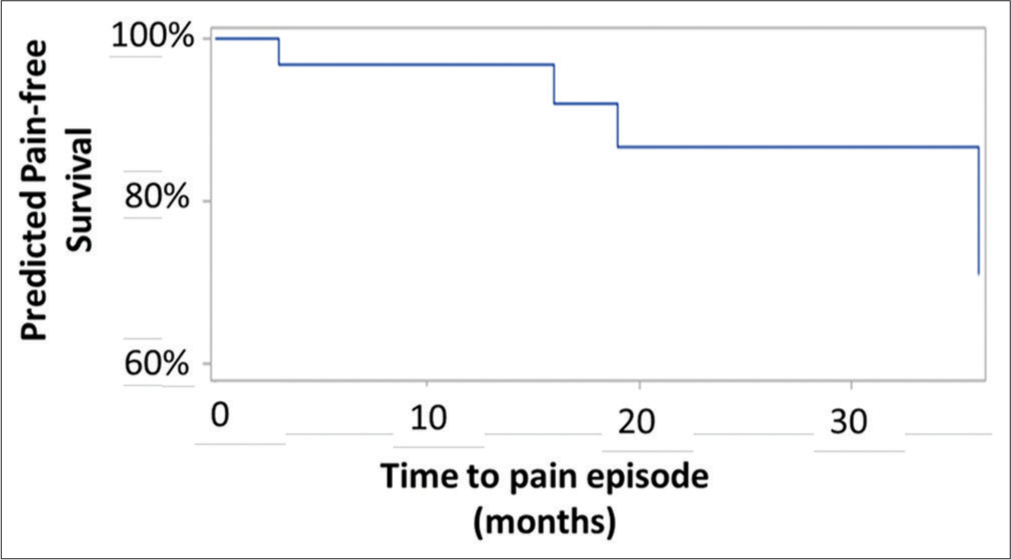
At last follow-up, significant pain relief was noted by 86.6% of patients (good plus excellent results). Relapse in pain was noted in four patients at 3, 16, 19, and 36 months. In two of them, the pain was similar to the pain before the radiosurgery procedure. One patient returned to the pain-free state with medication; although his pain presents occasionally, it is not as intense as previously recorded. One patient decided to undergo surgery; therefore, the case was considered a treatment failure.
Univariate analysis did not identify any factors associated with pain-free survival.
Of the 30 patients, seven patients continue under medication at the last follow-up. One of them, an 83-year-old patient suffering from TN for 19 years, does not want to leave the medication even in the absence of pain.
Nine patients were treated in acute status, with pain the same day of the procedure and between 1 and 10 days before the procedure in 21 patients.
Regarding the adverse effects of radiosurgery, one patient reported moderate hypoesthesia in all three divisions, two patients had tickling sensations, and one patient reported hypoesthesia in V2, although in this case, the patient presented dysesthesias in the same region before treatment with radiosurgery.
DISCUSSION
Historical background
Radiosurgery has become an excellent treatment choice for patients who do not respond to medical therapy or other surgical procedures. In the management of the typical TN, most patients are suitable candidates for GKS which was demonstrated to be an effective and safe option.[
The concept of SRS was introduced by Lars Leksell, who in 1951 began performing clinical radiosurgery with a stereotactic X-ray beam in patients with essential TN. Leksell was the first to use radiosurgery for the treatment of functional disorders such as TN. Using a conventional stereotactic frame, he directed the radiation beam produced by an orthovoltage X-ray tube at the TG.[
After treating a series of 40 patients, the Leksell team abandoned this approach because Leksell discovered the efficacy of the glycerol injection. This injection was first used for the visualization of the Meckel cave but turned out to be sufficient to stop the pain in a significant percentage of patients.[
Lindquist et al.[
Many years after Leksell’s report, Rand et al.[
Chen et al.[
Target location, accuracy, and treatment dose
Since the duration of pain relief tends to lessen over time, various modifications of the radiosurgical technique for TN have been devised to improve outcomes such as the duration of pain relief.[
Many authors have reported that higher the applied dose of the trigeminal nerve, the better the pain control, as well as greater benefits with the use of two isocenters.[
Accuracy is a critical aspect when placing isocenters on the trigeminal nerve. However, there are inaccuracies that depend on the images, the selection of nerve target site, mechanical errors of the stereotactic frame, and mechanical errors of the radiation equipment used.[
In our study, we applied the same doses used in the trigeminal nerve to the TG and we used two isocenters which were determined by the semioval shape of the ganglion and the adjacent fibers of the nerve similar to what was previously described to completely cover the TG.[
The dose is one of the most important factors that improve outcomes following GKS for TN. GKS is associated with high rates of pain control. Patients report excellent or good pain relief in more than 70% of cases.[
Pollock et al.,[
There is no dose established to be used on the TG. Chen et al.[
On the other hand, vascular injury has been reported in vessels adjacent to the entry zone. Pollock et al.[
More recently, Chen et al.[
It has been established that radiosurgery should not be performed on patients under acute attacks of pain, due to the known latency necessary to produce pain relief with radiosurgery and the fact that it often takes several weeks or months to reach the optimal therapeutic peak. Thus, radiosurgery is considered a second option for patients with severe pain, with difficulty speaking or maintaining adequate hydration, who need an immediate method of pain control through percutaneous procedures.[
Complications, recurrence, and latency period
For excellent results, radiosurgery and other ablative techniques must find a balance between the production of an injury to trigeminal fibers that are sufficient to provide adequate pain relief without causing so much damage that it produces complications such as facial numbness, paresthesias, bothersome dysesthesia, masseter weakness, or disagreeable numbness that could transform into anesthesia Dolorosa.[
With radiosurgery targeting the trigeminal nerve, sensory dysfunction is less common than with ablative techniques, ranging between 6% and 66%,[
In our series, the percentage of complications during the follow-up period was very low, even taking into account that the maximum dose used can be considered within the high range.
Another important aspect to consider is the recurrence of attacks of facial pain which is contrary to the goal of obtaining a pain-free status.[
The effect of the latency period after SRS is reported in most series as an interval of 1–2 months.[
It has been suggested that this parameter should be seriously considered for radiosurgical planning. Patients with previous surgery seem to be more frequently late responders to GKS than patients with no history of previous surgery.[
In our cases, we observed a short latency period with a median of 7 days (range, 1–40). In 18 patients, the pain relief was obtained before 7 days. Patients with a long history of TN had similar responses to those with a short history; history of previous surgery did not have any influence on the results.
The TG role of the satellite glial cells (SGC)
Although the vascular compression theory is popular, it cannot account for all phenomena associated with TN.[
It seems that the main feature of TN pain is its dynamic nature which is difficult to explain in purely anatomical terms. The fact that the pain is not continuous, but paroxysmal speaks against the fact that a simple compression is an ectopic generator of pain at the level of the lesion. Moreover, pain must not only occur spontaneously but must also be produced, as often happens, by innocuous tactile stimuli. Therefore, demyelination alone does not provide clear evidence of the characteristic symptoms of the disease. It has been assumed that the pain is the result of hyperactivity or abnormal discharges. These arise from the gasserian ganglion, the “injured” nerve root, and the trigeminal nucleus within the brainstem.[
TG seems to have a predominant role given its cellular architecture. The crescent-shaped TG lies on Meckel’s cave, which is a rigid structure. The TG has dimensions ranging from 14 to 22 mm in length and 4–5 mm in thickness and is easily identified on CT or MRI.[
The fine structure of the TG contains cells that give rise to the three divisions of the trigeminal nerve. The cell bodies of neurons are completely surrounded by specialized glial cells known as SGC that together form distinct, functional units.[
Neurons and SGCs extend processes that are thought to facilitate the exchange of chemicals between neurons and glia and can communicate directly through gap junctions that allow for the direct transfer of small molecular weight molecules, such as ions, that regulate cellular excitability, metabolic precursors, and second messengers.[
V1, V2, and V3 regions are interconnected. Stimulation of V3 neurons could cause increased levels of active signaling proteins in neuronal and SGCs in other regions of the ganglion which could explain why a pain originating in V2, for example, is propagated to V1 and/or V3, contributing to signal propagation and chronic pain.[
SGCs divide after an insult to the peripheral nerve including nerve damage.[
Therefore, the glial cells have the ability to communicate with neurons and modulate their activity, particularly in TG where SGCs establish a privileged relationship with the surrounding neuronal bodies. The increased neuron-glial interactions are thought to play an important role in the induction and maintenance of peripheral sensitization of trigeminal nociceptors.[
TG may become the first level of the pathophysiological changes of modulation of afferent signaling, as it allows the interaction between different types of information and seems to be a trigger of the central sensitization mechanism as observed also in the spinal cord dorsal horn neurons in relation to the spinal ganglion.[
Thus, knowledge about the SGC and its mechanisms of interaction with the neuronal body assumes a growing importance in the search for new targets for chronic pain treatment, which includes TN.[
The occurrence of a refractory period of seconds to minutes after an attack of TN is well known, during which further attacks cannot be provoked. Besides demyelination, other factors could delay the restoration of membrane potentials and excitability after an episode of TN.[
The refractoriness period could be explained by the release of potassium ions due to the activation of potassium channels by calcium, leading to neuronal hyperpolarization and the trigger stop.[
Our findings suggest that there is a longer refractory period as a consequence of the treatment of the ganglion and nerve adjacent fibers, probably affecting primarily the SGCs and, as a consequence, the repolarization process which would produce a delay of a possible new attack.
How does radiosurgery act on trigeminal pain?
At present, from a pathological point of view, the exact mechanism of pain relief is unknown. There is the assumption that there exists a critical region in which the central myelin (oligodendrocyte) becomes peripheral (Schwann cell) myelin. The oligodendrocytes are more sensitive to irradiation than Schwann cells, and consequently, a stronger radiobiological effect would occur on the root entry zone.[
However, Régis et al. treated the trigeminal nerve at the pontine cistern where the myelin is peripheral (Schwan cells) obtaining the same results.[
In any case, after the treatment, it is observed that patients report an immediate decrease in the intensity of pain even if the attacks still occur. This is postulated to be the result of an immediate interruption of ephaptic transmission. Several weeks later, there is a complete cessation of the attacks. This is probably secondary to delayed demyelination injury to the nerve. The compact union of fibers from different divisions would facilitate the irradiation of the entire nerve with the smallest volume of energy (4-mm collimator).[
There is a growing body of evidence that glial satellite cells undergo structural and biochemical changes after nerve injury which influence neuronal excitability and consequently the development and/or maintenance of pain in different animal models of chronic pain.[
CONCLUSIONS
Radiosurgery on TG for the treatment of TN has several positive aspects: short latency period, minor collateral effects, a high percentage of pain control, and the pain relief which is not related to the time of the diagnosis of TN. In general, a better understanding of the radiobiology and pathophysiology of NT is important to explain the mechanism of action of radiosurgery.
The current series investigates the use of TG to treat NT using GK with commonly used doses and high-resolution images. A comparative prospective randomized trial is necessary for the use of GKR on the TG for idiopathic TN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Aknowledgement
We thank Dr. Roman Liscak who provided insight and expertise for reviewing and for his comments that greatly improved the manuscript, although he may not agree with all of the interpretations/conclusions of this paper.
References
1. Alpert TE, Chung CT, Mitchell LT, Hodge CJ, Montgomery CT, Bogart JA. Gamma knife surgery for trigeminal neuralgia: Improved initial response with two isocenters and increasing dose. J Neurosurg. 2005. p. 185-8
2. Chen JC, Chao K, Rahimian J. De novo superior cerebellar artery aneurysm following radiosurgery for trigeminal neuralgia. J Clin Neurosci. 2017. 38: 87-90
3. Chen MJ, Shao ZY, Zhang WJ, Wang ZH, Zhang WH, Hu HS. X-knife stereotactic radiosurgery on the trigeminal ganglion to treat trigeminal neuralgia: A preliminary study. Minim Invasive Neurosurg. 2010. 53: 223-8
4. Costa FA, Moreira Neto FL. Satellite glial cells in sensory ganglia: Its role in pain. Rev Bras Anestesiol. 2015. 65: 73-81
5. Dellaretti M, Reyns N, Touzet G, Sarrazin T, Dubois F, Lartigau E. Clinical outcomes after gamma knife surgery for idiopathic trigeminal neuralgia: Review of 76 consecutive cases. J Neurosurg. 2008. p. 173-8
6. Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: The ignition hypothesis. Clin J Pain. 2002. 18: 4-13
7. Dhople AA, Adams JR, Maggio WW, Naqvi SA, Regine WF, Kwok Y. Long-term outcomes of gamma knife radiosurgery for classic trigeminal neuralgia: Implications of treatment and critical review of the literature. Clinical article. J Neurosurg. 2009. 111: 351-8
8. Flickinger JC, Pollock BE, Kondziolka D, Phuong LK, Foote RL, Stafford SL. Does increased nerve length within the treatment volume improve trigeminal neuralgia radiosurgery? A prospective double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2001. 51: 449-54
9. Fountas KN, Smith JR, Lee GP, Jenkins PD, Cantrell RR, Sheils WC. Gamma knife stereotactic radiosurgical treatment of idiopathic trigeminal neuralgia: Long-term outcome and complications. Neurosurg Focus. 2007. 23: E8-
10. Gorgulho A. Radiation mechanisms of pain control in classical trigeminal neuralgia. Surg Neurol Int. 2012. 3: S17-25
11. Grasso G, Landi A, Alafaci C. A novel pathophysiological mechanism contributing to trigeminal neuralgia. Mol Med. 2016. 22: 452-4
12. Haines SJ, Jannetta PJ, Zorub DS. Microvascular relations of the trigeminal nerve. An anatomical study with clinical correlation. J Neurosurg. 1980. 52: 381-6
13. Håkanson S. Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery. 1981. 9: 638-46
14. Hanani M. Satellite glial cells in sensory ganglia: From form to function. Brain Res Brain Res Rev. 2005. 48: 457-76
15. Hasegawa T, Kondziolka D, Spiro R, Flickinger JC, Lunsford LD. Repeat radiosurgery for refractory trigeminal neuralgia. Neurosurgery. 2002. 50: 494-500
16. Herman JM, Petit JH, Amin P, Kwok Y, Dutta PR, Chin LS. Repeat gamma knife radiosurgery for refractory or recurrent trigeminal neuralgia: Treatment outcomes and quality-of-life assessment. Int J Radiat Oncol Biol Phys. 2004. 59: 112-6
17. Kanner AA, Neyman G, Suh JH, Weinhous MS, Lee SY, Barnett GH. Gamma knife radiosurgery for trigeminal neuralgia: Comparing the use of a 4-mm versus concentric 4-and 8-mm collimators. Stereotact Funct Neurosurg. 2004. 82: 49-57
18. Kim YH, Kim DG, Kim JW, Kim YH, Han JH, Chung HT. Is it effective to raise the irradiation dose from 80 to 85 gy in gamma knife radiosurgery for trigeminal neuralgia. ? Stereotact Funct Neurosurg. 2010. 88: 169-76
19. Kondziolka D, Lunsford LD, Flickinger JC, Young RF, Vermeulen S, Duma CM. Stereotactic radiosurgery for trigeminal neuralgia: A multiinstitutional study using the gamma unit. J Neurosurg. 1996. 84: 940-5
20. Kondziolka D, Perez B, Flickinger JC, Habeck M, Lunsford LD. Gamma knife radiosurgery for trigeminal neuralgia: Results and expectations. Arch Neurol. 1998. 55: 1524-9
21. Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand. 1971. 137: 311-4
22. Lindquist C, Kihlström L, Hellstrand E. Functional neurosurgery-a future for the gamma knife. ? Stereotact Funct Neurosurg. 1991. 57: 72-81
23. Little AS, Shetter AG, Shetter ME, Bay C, Rogers CL. Long-term pain response and quality of life in patients with typical trigeminal neuralgia treated with gamma knife stereotactic radiosurgery. Neurosurgery. 2008. 63: 915-23
24. Love S, Coakham HB. Trigeminal neuralgia: Pathology and pathogenesis. Brain. 2001. 124: 2347-60
25. Maesawa S, Salame C, Flickinger JC, Pirris S, Kondziolka D, Lunsford LD. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2001. 94: 14-20
26. Maher CO, Pollock BE. Radiation induced vascular injury after stereotactic radiosurgery for trigeminal neuralgia: Case report. Surg Neurol. 2000. 54: 189-93
27. Majoie CB, Hulsmans FJ, Verbeeten B, Castelijns JA, van Beek EJ, Valk J. Trigeminal neuralgia: Comparison of two MR imaging techniques in the demonstration of neurovascular contact. Radiology. 1997. 204: 455-60
28. Massager N, Lorenzoni J, Devriendt D, Desmedt F, Brotchi J, Levivier M. Gamma knife surgery for idiopathic trigeminal neuralgia performed using a far-anterior cisternal target and a high dose of radiation. J Neurosurg. 2004. 100: 597-605
29. Massager N, Murata N, Tamura M, Devriendt D, Levivier M, Régis J. Influence of nerve radiation dose in the incidence of trigeminal dysfunction after trigeminal neuralgia radiosurgery. Neurosurgery. 2007. 60: 681-7
30. Matsuda S, Serizawa T, Sato M, Ono J. Gamma knife radiosurgery for trigeminal neuralgia: The dry-eye complication. J Neurosurg. 2002. 97: 525-8
31. Morbidini-Gaffney S, Chung CT, Alpert TE, Newman N, Hahn SS, Shah H. Doses greater than 85 gy and two isocenters in gamma knife surgery for trigeminal neuralgia: Updated results. J Neurosurg. 2006. p. 107-11
32. Mousavi SH, Niranjan A, Huang MJ, Laghari FJ, Shin SS, Mindlin JL. Early radiosurgery provides superior pain relief for trigeminal neuralgia patients. Neurology. 2015. 85: 2159-65
33. Nicol B, Regine WF, Courtney C, Meigooni A, Sanders M, Young B. Gamma knife radiosurgery using 90 gy for trigeminal neuralgia. J Neurosurg. 2000. 93: 152-4
34. Ohara PT, Vit JP, Bhargava A, Romero M, Sundberg C, Charles AC. Gliopathic pain: When satellite glial cells go bad. Neuroscientist. 2009. 15: 450-63
35. Park SH, Hwang SK, Kang DH, Park J, Hwang JH, Sung JK. The retrogasserian zone versus dorsal root entry zone: Comparison of two targeting techniques of gamma knife radiosurgery for trigeminal neuralgia. Acta Neurochir (Wien). 2010. 152: 1165-70
36. Pollock BE, Phuong LK, Foote RL, Stafford SL, Gorman DA. High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery. 2001. 49: 58-62
37. Pollock BE, Phuong LK, Gorman DA, Foote RL, Stafford SL. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2002. 97: 347-53
38. Rand RW, Jacques DB, Melbye RW, Copcutt BG, Levenick MN, Fisher MR. Leksell gamma knife treatment of tic douloureux. Stereotact Funct Neurosurg. 1993. 61: 93-102
39. Régis J, Bartolomei F, Metellus P, Rey M, Genton P, Dravet C. Radiosurgery for trigeminal neuralgia and epilepsy. Neurosurg Clin N Am. 1999. 10: 359-77
40. Régis J, Metellus P, Hayashi M, Roussel P, Donnet A, Bille-Turc F. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J Neurosurg. 2006. 104: 913-24
41. Régis J, Tuleasca C, Resseguier N, Carron R, Donnet A, Gaudart J. Long-term safety and efficacy of gamma knife surgery in classical trigeminal neuralgia: A 497-patient historical cohort study. J Neurosurg. 2016. 124: 1079-87
42. Sabalys G, Juodzbalys G, Wang HL. Aetiology and pathogenesis of trigeminal neuralgia: A comprehensive review. J Oral Maxillofac Res. 2013. 3: e2-
43. Singh R, Davis J, Sharma S. Stereotactic radiosurgery for trigeminal neuralgia: A retrospective multi-institutional examination of treatment outcomes. Cureus. 2016. 8: e554-
44. Somaza S, Hurtado W, Montilla E, Ghaleb J. Gamma knife radiosurgery to the trigeminal ganglion for treatment of trigeminal neuralgia secondary to vertebrobasilar ectasia. Surg Neurol Int. 2014. 5: S580-5
45. Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009. 33: 784-92
46. Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007. 47: 1008-23
47. Uchikawa H, Nishi T, Kaku Y, Goto T, Kuratsu JI, Yano S. Delayed development of aneurysms following gamma knife surgery for trigeminal neuralgia: Report of 2 cases. World Neurosurg. 2017. 99: 813.e13-21
48. Xu Z, Schlesinger D, Moldovan K, Przybylowski C, Sun X, Lee CC. Impact of target location on the response of trigeminal neuralgia to stereotactic radiosurgery. J Neurosurg. 2014. 120: 716-24
49. Young B, Shivazad A, Kryscio RJ, St Clair W, Bush HM. Long-term outcome of high-dose γ knife surgery in treatment of trigeminal neuralgia. J Neurosurg. 2013. 119: 1166-75
50. Young RF, Vermeulen SS, Grimm P, Blasko J, Posewitz A. Gamma knife radiosurgery for treatment of trigeminal neuralgia: Idiopathic and tumor related. Neurology. 1997. 48: 608-14
51. Yousry I, Moriggl B, Schmid UD, Naidich TP, Yousry TA. Trigeminal ganglion and its divisions: Detailed anatomic MR imaging with contrast-enhanced 3D constructive interference in the steady state sequences. AJNR Am J Neuroradiol. 2005. 26: 1128-35