- Hassan 2 University, Faculty of Medicine and Pharmacy, Casablanca, Morocco
- Department of Neurosurgery, IBN Rochd University Hospital Center, Casablanca, Morocco
Correspondence Address:
Mohammed Yassine HAOUAS, Hassan 2 University, Faculty of Medicine and Pharmacy Casablanca, Department of Neurosurgery, UHC Ibn Rochd, Casablanca, Morocco.
DOI:10.25259/SNI_327_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammed Yassine HAOUAS1,2, Amine ELKHAMOUYE1,2, Khalid AADOUD1,2, Abdelkouddous LAAIDI1,2, Khadija IBAHIOIN1,2, Said HILMANI1,2, Abdelhakim LAKHDAR1,2. Giant intracranial tuberculomas in children: An unexpected diagnosis and difficult management – About two cases and review of the literature. 25-Oct-2024;15:378
How to cite this URL: Mohammed Yassine HAOUAS1,2, Amine ELKHAMOUYE1,2, Khalid AADOUD1,2, Abdelkouddous LAAIDI1,2, Khadija IBAHIOIN1,2, Said HILMANI1,2, Abdelhakim LAKHDAR1,2. Giant intracranial tuberculomas in children: An unexpected diagnosis and difficult management – About two cases and review of the literature. 25-Oct-2024;15:378. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13168
Abstract
Background:Giant intracranial tuberculomas are rare space-occupying lesions in the brain parenchyma, with a diameter >2.5 cm. They can mimic gliomas, meningiomas, and metastases. Diagnosis of this disease can be difficult without histological evidence. Tuberculosis (TB) affects people of all ages but is a major health problem among children. Misdiagnosis is common, as many clinical and radiological features are non-specific.
Case Description:Case 1: A 4-year-old child presented with intracranial hypertensive syndrome and Brave– Jackson seizures. Imaging showed a left temporoparietal lesion with intense peripheral ring enhancement after gadolinium injection, and attaching to the dura mater. Total surgical excision was performed, and histological analysis confirmed granulomatous TB. A month later, he presented to the emergency department with neurological deterioration. Magnetic resonance imaging revealed disseminated TB of the central nervous system, with tuberculomas in the brain stem. The child died after a month in intensive care. Case 2: An 11-year-old boy presented with a headache that had been progressively worsening for 7 months. Imaging revealed a right frontal process mimicking a high-grade glial tumor. The child underwent surgery with total excision of the tumor. After a few days, he developed tubercular miliaria and was put on anti-bacillary treatment.
Conclusion:Treatment includes antituberculosis therapy combined with surgery. This article describes the value of surgery for giant intracranial tuberculomas in two children under our care, with a review of the literature. We believe that the results of surgery for giant intracranial tuberculomas in children are favorable.
Keywords: Brain tumor, Children, Extrapulmonary tuberculosis, Giant intracranial tuberculoma, Pediatric
INTRODUCTION
Tuberculosis (TB) is a common disease in developing countries caused by Mycobacterium tuberculosis bacteria. Meningitis is by far the most common manifestation of central nervous system (CNS) TB CNS.[
Giant intracranial tuberculomas are defined as space occupying lesions >2 cm in diameter by some authors and 2.5 cm by others.[
The natural history of tuberculomas spectrum encompasses complete resolution with anti-TB medications to severe neurological disability from mass effect to tuberculoma, meningeal involvement with basilar arachnoiditis, and development of hydrocephalus. Although there are studies about the medical treatment of tuberculomas, there are no well-established guidelines for whether medical or surgical treatment is best in achieving the resolution of symptoms and preventing further neurological complications.[
Surgical treatment is widely used for this form of TB, associated with medical treatment with anti-TB drugs and corticoids.
In this article, we report two cases of giant intracranial tuberculomas surgically treated in two children to highlight the diagnostic and therapeutic difficulties of this pathology in this age group.
CASE PRESENTATION
Case 1
A 4-year-old boy with a history of medically treated fistulized cervical soft-tissue collection with good progression presented with intracranial hypertensive syndrome and Brave–Jacksonian seizures and fever. Imaging showed a left temporoparietal lesion with intense peripheral ring enhancement after gadolinium injection and attaching to the dura mater, mimicking a meningioma [
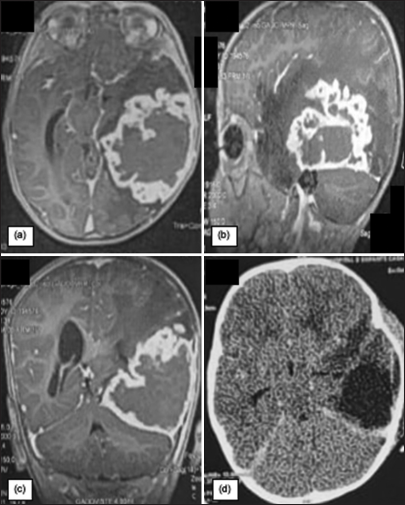
Figure 1:
Preoperative magnetic resonance imaging with (a) Axial, (b) Coronal, and (c) Sagittal T1 sequences with gadolinium injection, showing a tumor process measuring 63 mm wide/78 mm in diameter anteroposterior/60 mm in height, which attaches to the dura mater mimicking a giant meningioma. Axial sections of the control cerebral computed tomography scan (d) Show complete removal of the tumor.
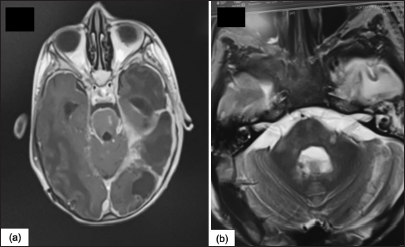
A month later, with a well-administered anti-bacillary treatment in accordance with our country’s national TB control program (Rifampicin, isoniazid, pyrazinamide and ethambutol, for the first 2 months/rifampicin, and isoniazid for the next 10 months), he presented to the emergency department with neurological deterioration. A cerebral CT scan showed hydrocephalus, which was shunted. Magnetic resonance imaging (MRI) revealed disseminated TB of the central nervous system, with tuberculomas in the brain stem [
Case 2
An 11-year-old boy with no specific pathological history presented with an intracranial hypertension syndrome of progressively worsening headache for 7 months, without motor or sensory deficits or seizures. The evolution of the disease was in a context of apyrexia with unchecked weight loss. The rest of the clinical examination was unremarkable. Biological tests were normal.
Cerebral CT revealed a right frontal intra-axial process that enhanced after contrast injection in an annular fashion and was surrounded by true perilesional edema, causing subfalcoral involvement and non-active exclusion hydrocephalus [
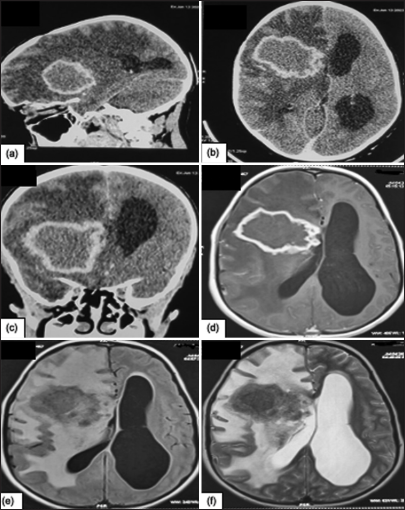
Figure 4:
Cerebral computed tomography scan, (a) Sagital section and (b) Axial section shows a right frontal intra-axial lesion, roughly rounded with irregular contour, well-limited, lobulated, iso-dense in relation to the cortex, taking contrast with annular resurfacing with diffuse hypodensity linked to perilesional edema exerting a mass effect on the right lateral ventricle with exclusion hydrocephalus. (c) On coronal sections of brain scans, the intra-axial process can be seen, with a mass effect on the homolateral ventricle. More precise magnetic resonance imaging shows the right frontal intra-axial lesion, roughly rounded with an irregular, well-lobulated contour. (d) T1 hyposignal, (e) Fluid attenuated inversion recovery hyposignal, (f) T2 heterogeneous, Annular gadolinium uptake with diffuse perilesional edema exerting a mass effect on the right lateral ventricle with the exclusion of hydrocephalus.
The data collected thus far leaned toward the diagnosis of glial tumors, although not definitively confirmed, with other potential diagnoses, such as TB, also being considered. Consequently, surgery was planned for the patient.
Intraoperatively, following a craniotomy and opening of the dura mater, the entire mass was excised in a single piece measuring 6 cm in diameter, thanks to the clear cleavage plane between the tumor and the parenchyma. Histological analysis confirmed granulomatous TB [
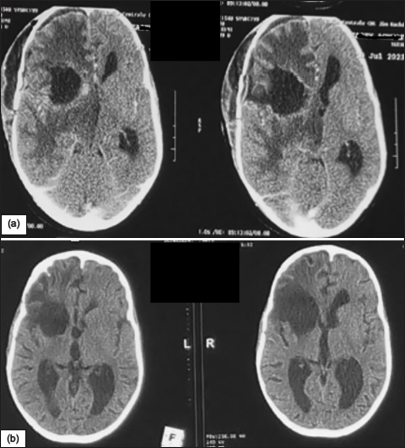
The patient declared that she was unaware that she had TB and denied any contact with TB patients; the chest X-ray taken as part of the pre-anesthetic work-up was normal. The patient was then treated for 12 months with rifampicin, isoniazid, pyrazinamide, and ethambutol for the first 2 months. At follow-up, the patient showed a marked improvement in headache but developed a dry cough after 1 month’s treatment, with chest X-ray findings of tuberculous pleuropneumopathy. This cough regressed spontaneously after continuation of anti-TB treatment, with normal chest radiology after 4 months of treatment. The follow-up CT scan after 6 months of treatment was satisfactory [
DISCUSSION
Giant intracranial tuberculomas are infrequent CNS lesions that form due to hematogenous dissemination of M. tuberculosis from a distant source. Tuberculomas are commonly seen in developing countries, while the incidence in developed countries has decreased over the past century. Tuberculomas have been reported as being up to 10% of CNS masses in countries with a high prevalence of TB. Despite being a common lesion in areas with endemic TB, intracranial tuberculomas are estimated to occur in only 0.2– 4.7% of all CNS TB cases. This low incidence of intracranial tuberculomas presents a major problem in the pediatric population. With CNS TB being <2% of all reported TB cases and intracranial tuberculomas being a fraction of that, locating an exact percentage of pediatric patients affected is tough. Although finding an exact percentage of pediatric patients affected is difficult, it has been estimated that over 50% of all tuberculomas occur in individuals <20 years old. This high prevalence of tuberculomas in the pediatric population is a major concern given the difficulty in diagnosis and higher mortality rates in this age group.
Tuberculomas may be the only form of TB in the child. It occurs mostly in immunocompetent children, compared to other forms of CNS TB, mainly because in a healthy individual with a good immune response, the infection is better contained. This is in contrast to patients with poor cellular immunity and is seen in immunocompromised states such as malnutrition, diabetes, neoplastic diseases, chemotherapy, immunosuppressive drug therapy, and HIV infection, which account for the majority of cases for all forms of CNS TB, including meningitis and tuberculomas.[
Diagnostic evaluation is crucial to the formulation of a management plan for CNS tuberculomas. There are no pathognomonic clinical features of CNS tuberculomas, and making a diagnosis usually requires a high index of suspicion and the presence of suggestive clinical and epidemiologic features. Due to their varied and nonspecific clinical presentations, CNS tuberculomas are mistaken for other mass lesions, particularly brain tumors. The ability of modern neuroimaging, in particular CT and MRI, to visualize intracranial pathology has greatly facilitated diagnosis. Imaging now plays a central role in the diagnostic evaluation of patients suspected of having CNS tuberculomas.
Methods for diagnosing intracranial tuberculomas are based entirely on various imaging techniques due to nonspecific symptomatology and inconclusive laboratory findings. The brain MRI is the most sensitive test for detecting tuberculomas. Although tuberculomas can have a variable appearance, they are usually round or ovoid, well-circumscribed masses ranging in size from a few millimeters to several centimeters. They are often solitary but may be multiple. Most are isointense to the cerebral cortex on T1-weighted images and hyperintense on T2-weighted images, with surrounding edema. The rim-enhancing pattern on administration of gadolinium contrast is common. The central low-signal intensity with the use of gadolinium indicates caseation. Diffusion-weighted imaging is a novel MRI technique which can be helpful in differentiating tuberculomas from other lesions. They show restricted diffusion corresponding to high cellularity and are hyperintense on apparent diffusion coefficient maps. Another useful tool when faced with diagnostic ambiguity is magnetic resonance spectroscopy, which can be helpful in distinguishing tuberculomas from abscesses, cystic or necrotic tumors, or other inflammatory lesions. A lipid peak and a low N-acetyl aspartate peak usually characterize the tuberculomas. An advantage of using MRI is the lack of radiation involved and its ability to give a more detailed image of lesion morphology in comparison to CT.[
Children who present with intracranial space-occupying lesions in TB-endemic regions represent a diagnostic challenge. The differential diagnosis of intracranial tuberculoma in children is extensive and includes benign and malignant neoplasms, other mass lesions, abscesses, and other granulomatous diseases.[
Incorrect diagnosis is not uncommon and can have dire consequences, given that invasive treatment is usually contraindicated for other diseases. A solid diagnosis of tuberculoma is essential for guiding appropriate treatment and for giving an accurate prognosis to patients and their families. In view of the above imaging findings, it is essential to differentiate between tuberculoma and other lesions and to exclude disseminated disease. The clinical and imaging features of some diseases can closely mimic tuberculoma, and the case for tissue sampling or biopsy of the lesion is usually determined on these grounds.
The medical management of intracranial tuberculomas begins with the administration of antitubercular drugs. An appropriate regimen for pediatric patients is isoniazid 10–15 mg/kg (up to 300 mg/day), rifampin 10 mg/kg (up to 600 mg/day), ethambutol 15–25 mg/kg (up to 1500 mg/day), and pyrazinamide 35–40 mg/kg (up to 2 g/day) daily in divided doses for 2 months, followed by a regimen of isoniazid and rifampin daily or twice weekly for an additional 7–10 months. If culture data can be obtained, drug susceptibility testing should be performed and the regimen modified based on sensitivity results. Molecular techniques such as nucleic acid amplification assays and DNA probes may be useful in diagnosing M. tuberculosis and determining drug susceptibilities, but these newer tests lack sensitivity for tuberculomas and are mainly useful for defining the initiation phase of antitubercular therapy. Clinical improvement, with resolution of constitutional symptoms and neuroradiographic improvement, should be observed within 3–6 months.
The use of corticosteroids in the treatment of CNS tuberculomas is based on their ability to reduce vasogenic edema and mass effect in intracranial lesions, making it a useful adjunct to antitubercular drug therapy. Prednisone is the most commonly used corticosteroid, with the dose being tailored to the severity and location of the tuberculomas. The usual dose for moderate severity is 1 mg/kg/day for 2–4 weeks, followed by 1–2 weeks at 0.5 mg/kg/day or until neurologic symptoms begin to improve. High-dose prednisone therapy (4 mg/kg/day) should be considered for patients with deteriorating mental status or signs of impending herniation, with subsequent gradual tapering of the dosage as clinical improvement is observed. Dosages higher than 60 mg/day of prednisone have been associated with psychiatric side effects and insomnia, particularly in the elderly, but these side effects are less common in children.[
Surgery is employed in the following situations: when the diagnosis is uncertain, and neoplasm cannot be excluded; giant tuberculoma with imminent risk of cerebral involvement; when the tuberculoma is associated with hydrocephalus; and when there is progressive neurological deficit despite adequate medical treatment.
There are three main surgical approaches to the removal of cerebral tuberculomas. These are craniotomy techniques for larger lesions with significant mass effect and midline displacement, minimally invasive surgical options such as endoscopic approach for intraventricular tuberculomas, and biopsy and stereotactic aspiration for deep tuberculomas or those located in an eloquent area. Ventricular shunting is often indicated for hydrocephalus associated with tuberculoma.[
A craniotomy is an essential approach in the surgical treatment of tuberculomas, particularly in children who typically present with features of high intracranial tension. In general, surgery aims to obtain tissue for diagnosis and to reduce the size of the lesion. Giant tuberculomas of the posterior cerebral fossa should be excised. In most cases, craniotomy and excision of the tuberculoma can be performed without increased morbidity or mortality associated with medical treatment, but in some cases, the lesion may be too deep or located in an eloquent area to be safely excised, particularly in children, stereotactic or endoscopic approaches may be attempted. Endoscopy can be used to remove intraventricular tuberculomas, which are not accessible by any other means, with the advantages of minimally invasive surgery. Subependymal tuberculomas may also benefit from endoscopic surgery.
The different surgical approaches to tuberculomas can be varied and would depend on the neurosurgeon and available facility, as well as the patient’s overall condition, the tuberculomas size, and location.[
Long-term outcomes and follow-up care for children with intracranial tuberculomas have rarely been reported. Regular follow-up with clinical examination, imaging, and tuberculin skin testing is recommended for at least 2 years after the end of treatment, as little is known about late relapse rates. In our study, one child relapsed 3 months after surgery while on anti-TB treatment with a dramatic evolution. Patients whose clinical condition is stable or has improved by the time medical treatment is stopped can be followed up every 3–6 months for the first 2 years, then every year. Patients whose clinical condition is poor or whose neuroimaging results are advanced before treatment is stopped should be followed up more frequently.
However, it should be noted that long-term results comparing surgical treatment combined with anti-TB therapy of giant intracranial tuberculomas in children with medical treatment alone have never been directly described in a randomized trial but only examined in retrospective analyses of case series or case reports. The conclusions that can be drawn from these studies are limited by the great differences in patient selection and management from one center to another. Satyarthee reported an extra-giant tuberculoma with considerable mass effect in an 11-year-old child located in the frontal lobe, with a strong suspicion of a neoplastic mass. However, the patient refused surgery and was maintained on anti-TB treatment and corticoids. He reported radical improvement in 6 months.[
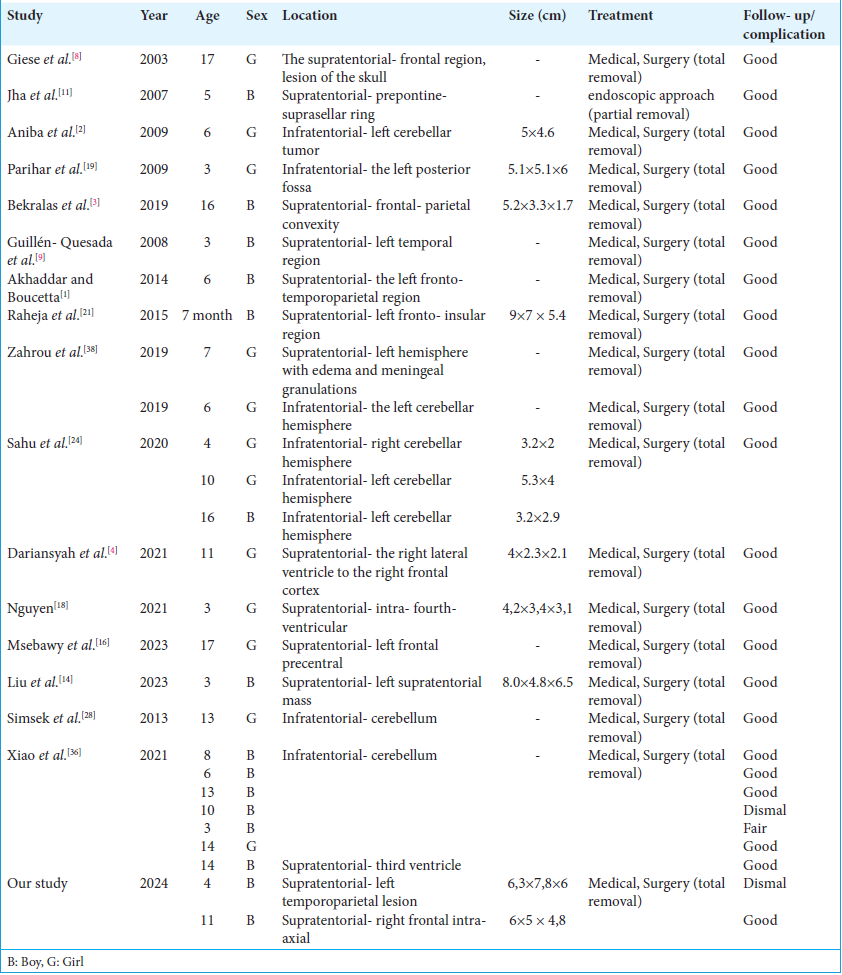
We carried out a review of the literature on giant pediatric intracranial tuberculoma treated surgically for removal, which included 24 children from 16 studies [
CONCLUSION
The management of giant intracranial tuberculomas in children is complex and sophisticated. Rapid decision-making is required to deal with an unexpected, life-threatening situation. Tuberculoma itself is a difficult pathology to manage, with a potential risk of acute deterioration due to the surrounding cerebral edema, resulting in high surgical morbidity and mortality rates. Management must be geared toward preventing an acute event due to mass effect or hydrocephalus and maintaining the patient’s good neurological condition. The broad application of medical and surgical management poses major challenges in terms of the decisions to be made. It is important to note the absence of expert guidelines for the management of this pathology. The decision taken must take into account the potential risks and benefits of each procedure, as there is no single strategy for these patients. In selected patients who respond well, surgery offers a treatment that enables immediate decompression of lesions with the aim of preventing an acute neurological event. Medication must always be continued throughout management, and corticosteroid titration is also important to prevent the paradoxical effects of this condition. Good communication between the attending physician, neurosurgeon, and pediatrician is necessary to monitor the effects of the intensive treatment administered to this patient. The results of this study reflect the management of this interesting and challenging condition.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akhaddar A, Boucetta M. Giant brain tuberculoma mimicking a malignant tumor in a child. Pan Afr Med J. 2014. 17: 112
2. Aniba K, Ghannane H, Jalal H, Belhaj Z, Ousehal A, Lmejjati M. Giant cerebellar tuberculoma mimicking a malignant tumor. Neurochirurgie. 2009. 55: 337-9
3. Bekralas H, Habchi N, Bouallag M, Boulaouad W, Oumchiche W, Djaafer M. Diagnosis error of a tuberculoma: Case report and review of the literature. J Neurosci Neurol Surg. 2019. 4: 1-2
4. Dariansyah AD, Suryaningtyas W, Parenrengi MA. Tuberculoma mimicking postoperative VP shunt seeding of craniopharyngioma: A rare case report. Surg Neurol Int. 2021. 12: 450
5. DeLance AR, Safaee M, Oh MC, Clark AJ, Kaur G, Sun MZ. Tuberculoma of the central nervous system. J Clin Neurosci. 2013. 20: 1333-41
6. Dubé MP, Holtom PD, Larsen RA. Tuberculous meningitis in patients with and without human immunodeficiency virus infection. Am J Med. 1992. 93: 520-4
7. Garg RK. Diagnosis of intracranial tuberculoma. Indian J Tuberc. 1996. 43: 35-9
8. Giese A, Kucinski T, Hagel C, Lohmann F. Intracranial tuberculomas mimicking a malignant disease in an immunocompetent patient. Acta Neurochir (Wien). 2003. 145: 513-7
9. Guillén-Quesada A, García-Armengol R, Pérez-Muñoz N, Gargallo E, García-García JJ, Costa-Clara JM. Intracranial tuberculoma: A case report and review of the literature. Rev Neurol. 2008. 47: 631-4
10. Henry M, Holzman RS, Rom WN, Garay SM, editors. Tuberculosis of the brain, meninges and spinal cord. Tuberculosis. Philadelphia, PA: Lippincot Williams and Wilkins; 2004. p.
11. Jha D, Khatri P, Choudhary A, Sethi R, Kumar S. Endoscopic third ventriculostomy in prepontine-suprasellar tuberculoma with tuberculous meningitis hydrocephalus: A case report. Pediatr Neurosurg. 2007. 43: 42-6
12. Khanna PC, Godinho S, Patkar DP, Pungavkar SA, Lawande MA. MR spectroscopy-aided differentiation: “Giant” extra-axial tuberculoma masquerading as meningioma. AJNR Am J Neuroradiol. 2006. 27: 1438-40
13. Khusro A, Aarti C. Extrapulmonary tuberculosis: An overview on infection beyond lungs. World News Nat Sci. 2020. 28: 131-41
14. Liu E, Kakodkar P, Pan H, Zhou A, Toyota P, Persad AR. Pediatric intracranial tuberculoma: Illustrative case. J Neurosurg Case Lessons. 2023. 6: CASE23236
15. Maheshwari V, Srivastava VK, Prasad S, Alam K. Tuberculoma-A significant diagnostic entity in brain biopsies of intracranial space occupying lesions in children. J Trop Pediatr. 2002. 48: 242-4
16. Msebawy AS, Al-Araji ZA, Nazar A, Alayyaf A, Saleh SA, Merie SM. Surgical resection of pericallosal tuberculoma through contralateral approach: A case report. Surg Neurol Int. 2023. 14: 396
17. Mukherjee S, Das R, Begum S. Tuberculoma of the brain-a diagnostic dilemma: Magnetic resonance spectroscopy a new ray of Hope. J Assoc Chest Phys. 2015. 3: 3-8
18. Nguyen MD. A giant fourth-ventricular tuberculoma mimicking a primary posterior fossa tumor. Asian Biomed (Res Rev News). 2021. 15: 191-5
19. Parihar V, Yadav YR, Sharma D. Giant extra-axial posterior fossa tuberculoma in a three-year-old child. Neurol India. 2009. 57: 218-20
20. Pohnán R, Hytych V, Holmquist I, Boštíková V, Doležel R, Ryska M. Increasing incidence of tuberculosis diagnosed by surgery: A single centre analysis in low-incidence country. Cent Eur J Public Health. 2020. 28: 48-52
21. Raheja A, Sinha S, Sable MN, Sharma MC, Sharma BS. A case of giant intracranial tuberculoma in an infant: Clinical and radiologic pitfalls. J Child Neurol. 2015. 30: 364-7
22. Rajshekhar V. Surgery for brain tuberculosis: A review. Acta Neurochir (Wien). 2015. 157: 1665-78
23. Rappaport ZH. Multiple intracranial tuberculomas with atypical response to tuberculostatic chemotherapy-review of the literature and own experience-comment. Acta Neurochir. 1997. 139: 202
24. Sahu C, Bhargava N, Singh V, Dwivedi P. Giant tuberculomas of brain: Rare neoplastic mimic. J Pediatr Neurosci. 2020. 15: 204-13
25. Satyarthee GD. Giant Intracerebral tuberculoma with complete disappearance on antitubercular therapy alone in a pediatric case: A case illustration with review of management strategy. J Pediatr Neurosci. 2017. 12: 180-4
26. Schaaf HS, Seddon JA. Management of tuberculous meningitis in children. Paediatr Int Child Health. 2021. 41: 231-6
27. Sharma V, Rajeshwari K, Kumar D, Gupta G. Clinicoepidemiological profile and prognostic factors in neurotuberculosis in children. Ann Child Neurol. 2023. 31: 103-12
28. Simsek H, Kutlay M, Colak A, Haholu A, Kaya H, Ozyurt M. Concomitant tubercular and fungal cerebellar abscess in an immunocompromised girl. Turk Neurosurg. 2013. 23: 88-94
29. Sonmez G, Ozturk E, Sildiroglu HO, Mutlu H, Cuce F, Senol MG. MRI findings of intracranial tuberculomas. Clin Imaging. 2008. 32: 88-92
30. Sumer S, Koktekir E, Demir NA, Akdemir G. Intracranial giant tuberculoma mimicking brain tumor: A case report. Turk Neurosurg. 2015. 25: 337-9
31. Swamy SB, Jaikumar V, Nagaraj NM, Khandelwal S. Intracranial giant tuberculoma: A 7-year institutional experience and literature review. Clin Neurol Neurosurg. 2023. 225: 107593
32. Thwaites GE, Schoeman JF. Update on tuberculosis of the central nervous system: Pathogenesis, diagnosis, and treatment. Clin Chest Med. 2009. 30: 745-54 ix
33. Trivedi R, Saksena S, Gupta RK. Magnetic resonance imaging in central nervous system tuberculosis. Indian J Radiol Imaging. 2009. 19: 256-65
34. Van Toorn R, Solomons R. Diagnosis and management of tuberculous meningitis in children-an update. Semin Pediatr Neurol. 2023. 47: 101071
35. Wasay M, Kheleani BA, Moolani MK, Zaheer J, Pui M, Hasan S. Brain CT and MRI findings in 100 consecutive patients with intracranial tuberculoma. J Neuroimaging. 2003. 13: 240-7
36. Xiao X, Li Q, Ju Y. Giant central nervous system tuberculoma in pediatric patients: Surgical case series. Childs Nerv Syst. 2021. 37: 2935-41
37. Yanardag H, Uygun S, Yumuk V, Caner M, Canbaz B. Cerebral tuberculosis mimicking intracranial tumour. Singapore Med J. 2005. 46: 731-3
38. Zahrou F, Elallouchi Y, Ghannane H, Benali SA, Aniba K. Diagnosis and management of intracranial tuberculomas: About 2 cases and a review of the literature. Pan Afr Med J. 2019. 34: 23