- Department of Neurosurgery, Ina Central Hospital, Ina, Japan
- Department of Neurosurgery, Aizawa Hospital, Matsumoto, Ina, Japan
- Department of Neurology, Ina Central Hospital, Ina, Japan
Correspondence Address:
Toshihiro Ogiwara, Department of Neurosurgery, Ina Central Hospital, Ina, Japan.
DOI:10.25259/SNI_876_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mana Wakabayashi1, Toshihiro Ogiwara1, Kiyoshi Ito2, Atsushi Sato1, Yoshiki Hanaoka1, Kenichi Kobayashi3, Yusaku Shimizu3, Kazuhiro Hongo1. Glioblastoma mimicking autoimmune meningitis in an adult: A complex diagnostic challenge. 21-Feb-2025;16:61
How to cite this URL: Mana Wakabayashi1, Toshihiro Ogiwara1, Kiyoshi Ito2, Atsushi Sato1, Yoshiki Hanaoka1, Kenichi Kobayashi3, Yusaku Shimizu3, Kazuhiro Hongo1. Glioblastoma mimicking autoimmune meningitis in an adult: A complex diagnostic challenge. 21-Feb-2025;16:61. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13391
Abstract
BackgroundGlioblastoma multiforme (GBM) is a highly aggressive primary brain tumor with a poor prognosis. It commonly affects the brain and rarely spreads outside the central nervous system owing to barriers like the blood–brain barrier. We present a rare case of GBM with atypical features mimicking autoimmune meningitis, complicating the diagnosis.
Case DescriptionA 45-year-old previously healthy man presented with persistent headaches, dizziness, vomiting, neck pain, diplopia from bilateral abducens nerve palsy, and cognitive dysfunction. The cerebrospinal fluid analysis did not confirm meningitis, although initial clinical and radiological findings suggested autoimmune meningitis. Head magnetic resonance imaging (MRI) showed postcontrast leptomeningeal enhancement and meningeal thickening. A spinal MRI revealed a contrast-enhancing lesion at the L1 level with leptomeningeal enhancement of the spinal cord. Despite empirical steroid therapy, his condition worsened, resulting in severe neurological deficits and impaired consciousness. A biopsy confirmed GBM through L1-2 laminectomy. Although adjuvant therapy was scheduled, his health rapidly declined, and he passed away on the 24th day of admission before receiving chemotherapy or radiotherapy.
ConclusionGBM can rarely present with noticeable symptoms and radiological features resembling autoimmune meningitis, posing diagnostic challenges. GBM cases involving spinal dissemination typically have a poorer prognosis, emphasizing the necessity of thorough diagnostic strategies. These should encompass histopathological biopsy and advanced imaging for optimal management, as delayed intervention significantly impacts survival. This report highlights the importance of maintaining a high level of suspicion for GBM and prompt intervention, including biopsy, in the presence of atypical clinical, radiological, and laboratory signs suggestive of meningitis.
Keywords: Atypical presentation, Autoimmune meningitis, Cerebrospinal fluid analysis, Glioblastoma multiforme, Spinal metastases
INTRODUCTION
Glioblastoma multiforme (GBM) is a prevalent malignant primary brain tumor, constituting 12– 15% of all intracranial neoplasms.[
CASE PRESENTATION
Initial presentation
A 45-year-old previously healthy man presented with a 1-week history of persistent headache and intermittent dizziness. He was diagnosed with migraine after a head computed tomography (CT) scan revealed no abnormal lesions. Sumatriptan was administered, but it proved ineffective as his symptoms progressed rapidly over 2 weeks, with new manifestations including vomiting, neck pain, speech disturbance, and diplopia caused by bilateral abducens nerve palsy. The patient was admitted to the Department of Neurology at our hospital for further assessment.
Neurological examination
On admission, neurological examination revealed cognitive dysfunction, sensory aphasia, and bilateral ataxia in the lower extremities. A stiff neck was observed, but the motor and sensory systems were intact.
Laboratory examination
Initial investigations revealed leukocytosis of 5.00 × 103/mL with 67% polymorphs, 28% lymphocytes, 5.4% monocytes, and 1.9% eosinophils. The results of tests for infectious disease, immunoglobulin, thyroid function, autoantibodies, and tumor markers were all normal or negative. Tests for antibodies against N-methyl-D-aspartate (NMDA), leucine-rich glioma inactivated 1 (LGI1), contactin-associated protein-like 2 (CASPR2), and myelin oligodendrocyte glycoprotein (MOG) were negative, indicating no evidence of immunodeficiency. Cerebrospinal fluid (CSF) analysis showed an opening pressure of 30 mmH2O and a cell count of 58/mm3, protein level of 1,310 mg/dL, and glucose level of 53 mg/dL. There were no increases in CSF adenosine deaminase (ADA), angiotensin converting enzyme (ACE), or soluble interleukin-2 receptor (sIL-2R) levels. CSF polymerase chain reaction (PCR), smears, cultures, and India ink preparations showed negativity for pathogens. Cytology revealed a predominance of lymphocytic inflammatory cells (Class II) with no atypical cells. Head magnetic resonance imaging (MRI) revealed hyperintense changes in the bilateral mesial temporal lobes, including the hippocampal gyrus and periventricular area surrounding both anterior horns of the lateral ventricles, as well as the choroid plexus of both lateral ventricles on Fluid attenuated inversion recovery (FLAIR) [
Figure 1:
(a and b) Axial images of the brain magnetic resonance imaging in Fluid attenuated inversion recovery (FLAIR) show hyperintense changes of the bilateral mesial temporal lobe and periventricular area surrounding both frontal horns of lateral ventricles, as well as choroid plexus. (c and d) Postcontrast leptomeningeal enhancement and thickening of meninges involving the brainstem can be seen.
Figure 2:
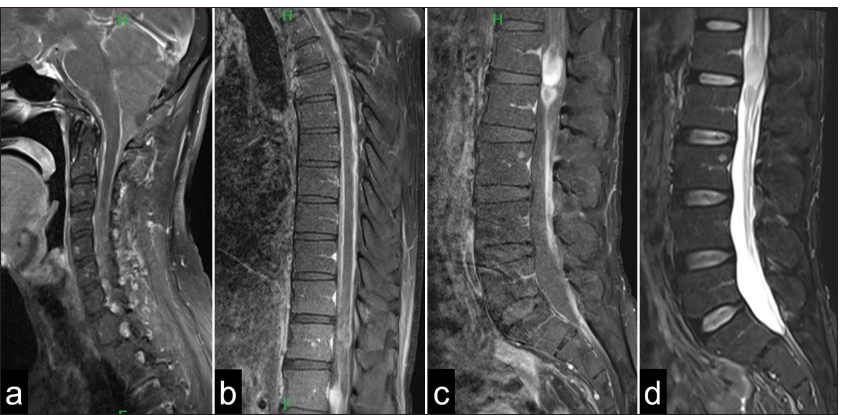
Sagittal magnetic resonance imaging of the (a) cervical spine, (b) thoracic spine, and (c and d) lumbar shows leptomeningeal thickening and postcontrast enhancement around the entire spinal cord. In addition, T2-weighted images reveal a (c) contrast-enhancing and (d) high-intensity mass lesion at the L1 level.
Diagnostic workup
Possible differential diagnoses at this time included inflammatory diseases of the CNS, such as autoimmune meningitis and herpes encephalitis, based on clinical symptoms, laboratory findings, and radiological findings, although CSF findings could not confirm meningitis.
Treatment and progression
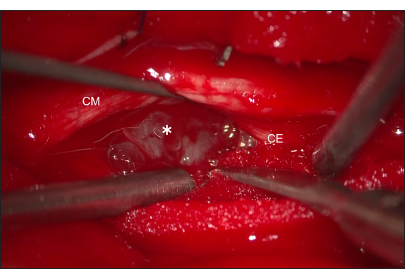
Empirical treatment for herpes encephalitis and autoimmune meningitis was initiated with antiviral medication (acyclovir 500 mg every 8 h for 2 weeks), and steroid pulse therapy (intravenous methylprednisolone 1000 mg for 3 days) was administered. Following steroid pulse therapy, the patient’s symptoms, including headache and nausea, temporarily improved; however, a few days later, they rapidly worsened, resulting in an inability to ingest food. The patient also reported severe neck and lower back pain. One week after the first steroid pulse therapy, a second course of steroid pulse therapy was administered following the same protocol. However, the patient showed no improvement, and his neurological condition continued to deteriorate. As the condition progressed, the patient developed bladder and rectal dysfunctions, accompanied by motor weakness in the bilateral lower extremities, which impaired his ability to stand and walk. At this stage, autoimmune meningitis was considered unlikely, and a biopsy of the spinal cord lesions was planned to obtain a definitive diagnosis. Thirteen days after admission, a biopsy of the spinal mass lesion was performed through L1–2 laminectomy. Upon dural opening, swelling of the conus medullaris and cauda equina was observed. A grayish, soft, hemorrhagic tumor was identified on the ventral side of the conus medullaris and cauda equina [
Figure 4:
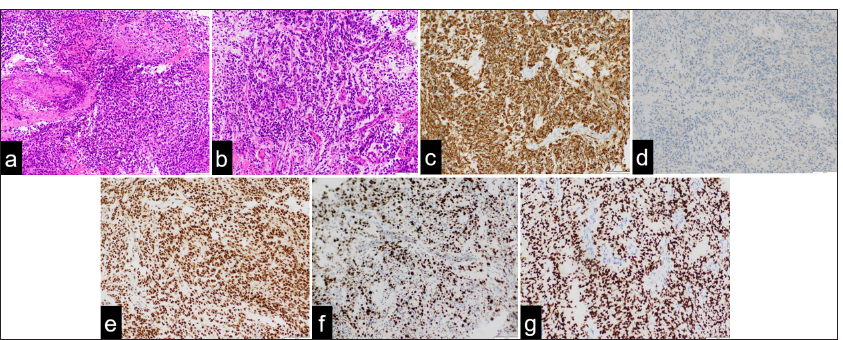
Histopathological findings demonstrate (a) medullary proliferation of tumor cells with variably shaped nuclei and numerous mitotic figures in hematoxylin and eosin (H and E) staining. (b) Diffuse malignant astroglia neoplasm with pseudopalisading patterns around areas of necrosis is observed with H and E staining. (c) Neoplastic cells show high mitotic activity and immunoexpression of glial fibrillary acidic protein. (d) Isocitrate dehydrogenase (IDH) staining reveals that the tumor is IDH wild-type, confirming the absence of IDH1 mutations. (e) Immunohistochemistry reveals strong nuclear staining for alpha-thalassemia/mental retardation (ATRX). (f) The Ki-67 immunolabeling index is approximately 60%. (g) Oligodendrocyte lineage transcription factor 2 (OLIG2) positivity indicates glial lineage. (Original magnification: ×20).
DISCUSSION
Mechanism of extracranial extension of GBM
Primary spinal GBM is a rare entity, and intracranial dissemination from the spinal GBM is extremely rare and has been reported infrequently.[
Glioblastoma mimicking autoimmune meningitis
GBM typically presents with irregular ring-like enhancement on postcontrast T1-weighted MRI, central necrosis, and surrounding edema. It causes a mass effect, infiltrates surrounding brain tissue, and may show restricted diffusion and vascular enhancement due to its high vascularity. On the other hand, MRI findings of meningitis typically include leptomeningeal enhancement on postcontrast T1-weighted images and mild cerebral edema on T2 and FLAIR images. There is usually no mass effect, although severe cases can show diffuse involvement of the meninges and brain parenchyma.
In this case, the atypical radiological findings deviated from typical characteristics of GBM, such as irregular margins, contrast enhancement, central necrosis, and extensive surrounding edema, which complicated the diagnosis.[
Learning points
We reported a case with an atypical clinical presentation of GBM mimicking autoimmune meningitis in an adult. It is difficult to establish a correct diagnosis without a biopsy in the initial stage because these pathologies share common clinical and radiological features. When some atypical or undoubted findings of meningitis or encephalitis are observed in laboratory examinations, including CSF analysis, the possibility of GBM should be considered even if autoimmune meningitis or viral encephalitis is radiologically diagnosed. Early definitive diagnosis and treatment are extremely important for malignancies such as GBM; therefore, a biopsy should be performed without hesitation to avoid misdiagnosis or delayed diagnosis.
CONCLUSION
Leptomeningeal diffuse GBM presents symptoms and imaging findings similar to those of autoimmune meningitis. The possibility of GBM should be considered if empirical treatment is ineffective, a definitive meningitis diagnosis is inconclusive from CSF examination, and symptoms deteriorate rapidly. GBM with spinal dissemination has a poor prognosis, necessitating prompt definitive diagnosis. Surgical biopsy for histopathological diagnosis should be promptly conducted without hesitation. Healthcare professionals managing CNS disorders must maintain high vigilance for timely diagnosis and effective management of these complex cases.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Angom RS, Nakka NM, Bhattacharya S. Advances in glioblastoma therapy: An update on current approaches. Brain Sci. 2023. 13: 1536
2. Asano N, Kitamura K, Seo Y, Mukai K, Soga T, Hondo H. Spinal cord glioblastoma multiforme with intracranial dissemination--case report. Neurol Med Chir (Tokyo). 1990. 30: 489-94
3. Bryan P. CSF seeding of intra-cranial tumours: A study of 96 cases. Clin Radiol. 1974. 25: 355-60
4. Chen J, Shi Q, Li S, Zhao Y, Huang H. Clinical characteristics of glioblastoma with metastatic spinal dissemination. Ann Palliat Med. 2022. 11: 506-12
5. Ginat DT, Schaefer PW. Imaging guidelines and findings of extracranial glioblastoma. J Neurooncol. 2014. 118: 9-18
6. Jang C, Cho BK, Hwang SH, Shin HJ, Yoon SH. Leptomeningeal spread at the diagnosis of glioblastoma multiforme: A case report and literature review. Brain Tumor Res Treat. 2022. 10: 183-9
7. Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016. 12: 1-13
8. Lawton CD, Nagasawa DT, Yang I, Fessler RG, Smith ZA. Leptomeningeal spinal metastases from glioblastoma multiforme: Treatment and management of an uncommon manifestation of disease. J Neurosurg Spine. 2012. 17: 438-48
9. Mandel JJ, Yust-Katz S, Cachia D, Wu J, Liu D, de Groot JF. Leptomeningeal dissemination in glioblastoma; an inspection of risk factors, treatment, and outcomes at a single institution. J Neurooncol. 2014. 120: 597-605
10. Shah A, Marianayagam NJ, Zamarud A, Park DJ, Persad AR, Soltys SG. Spinal metastases of pineal region glioblastoma with primitive neuroectodermal features highlighting the importance of molecular diagnoses: Illustrative case. J Neurosurg Case Lessons. 2023. 6: CASE23536
11. Sibanda Z, Farahani N, Ogbonnaya E, Albanese E. Glioblastoma multiforme: A rare case of spinal drop metastasis. World Neurosurg. 2020. 144: 24-7
12. Song D, Xu D, Gao Q, Hu P, Guo F. Intracranial metastases originating from pediatric primary spinal cord glioblastoma multiforme: A case report and literature review. Front Oncol. 2020. 10: 99
13. Stapińska-Syniec A, Rydzewski M, Acewicz A, KurkowskaJastrzębska I, Błażejewska-Hyżorek B, Sobstyl M. Atypical clinical presentation of glioblastoma mimicking autoimmune meningitis in an adult. Folia Neuropathol. 2022. 60: 250-6
14. Vertosick FT, Selker RG. Brain stem and spinal metastases of supratentorial glioblastoma multiforme: A clinical series. Neurosurgery. 1990. 27: 516-21 discussion 521
15. Witoonpanich P, Bamrungrak K, Jinawath A, Wongwaisayawan S, Phudhichareonrat S, Witoonpanich R. Glioblastoma multiforme at the corpus callosum with spinal leptomeningeal metastasis. Clin Neurol Neurosurg. 2011. 113: 407-10