- Department of Surgery, Aga Khan University Hospital, Karachi, Pakistan,
- Department of Neurosurgery, Vanderbilt University Medical Centre, Nashville, United States.
Correspondence Address:
Shahzad M. Shamim, Department of Surgery, Aga Khan University Hospital, Karachi, Pakistan.
DOI:10.25259/SNI_794_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zara Shah1, Saqib Kamran Bakhshi1, Mohammad Hamza Bajwa1, Mujtaba Khalil1, Michael C. Dewan2, Shahzad M. Shamim1. Human amniotic membrane as a dural substitute in neurosurgery: A systematic review. 04-Nov-2022;13:505
How to cite this URL: Zara Shah1, Saqib Kamran Bakhshi1, Mohammad Hamza Bajwa1, Mujtaba Khalil1, Michael C. Dewan2, Shahzad M. Shamim1. Human amniotic membrane as a dural substitute in neurosurgery: A systematic review. 04-Nov-2022;13:505. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11980
Abstract
Background: Several studies have highlighted the use of human amniotic membrane (HAM) in neurosurgical procedures as an effective dural substitute. HAM has inherent antifibrotic and anti-inflammatory properties and exhibits immunomodulatory effect that makes it an ideal dural substitute. Other advantages including easy availability, low cost of procurement, and storage also render it a promising dural substitute especially in low- and middle-income countries.
Methods: A systematic literature search was performed using PubMed, Scopus, and Google Scholar databases, using the search terms “human amniotic membrane,” “dural repair,” and “neurosurgery.” To be eligible for inclusion in our review, papers had to report primary data, be published in English language and report dural repair on humans with human amniotic membrane. Eligibility assessment was conducted by two independent reviewers with qualitative analysis on the basis of surgical utility, postoperative complications, and histological analysis.
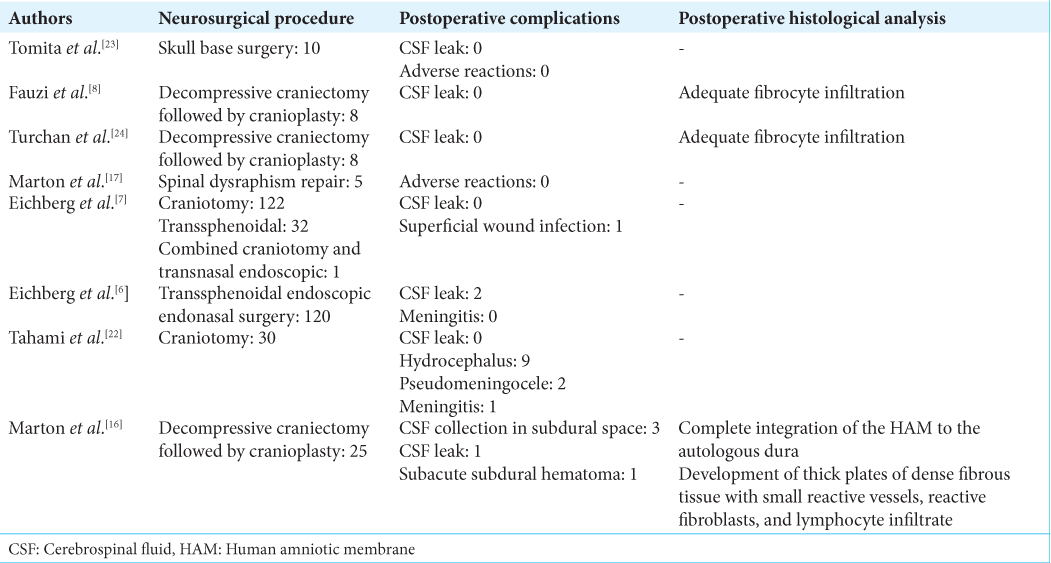
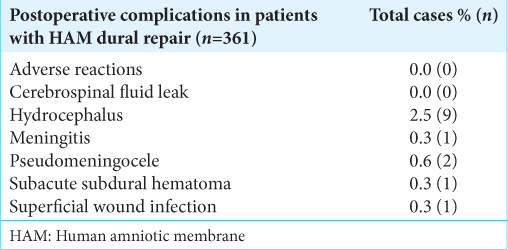
Results: Eight articles met the predefined inclusion criteria, including three randomized control trials and five cohort studies. We evaluated the use of HAM grafts in dural repair for elective cranial surgery (four studies), trauma surgery (three studies), and elective spine surgery (one study). Cases with postoperative cerebrospinal fluid (CSF) leak were reported by two studies. Other postoperative complications including meningitis, hydrocephalus, pseudomeningocele, CSF collection in subdural space, and subacute subdural hematoma were reported by one study each. Postsurgical histological analysis was reported by three studies highlighting the antiadhesive and integrative properties of HAM.
Conclusion: The current review of evidence suggests that in terms of postsurgical outcomes, HAM is comparable with commercially available dural substitutes.
Keywords: Amnion, Dural repair, Dural substitute, Duraplasty, Human amniotic membrane
INTRODUCTION
Human amniotic membrane (HAM) was first used for therapeutic purposes was for skin transplantation in 1910.[
For neurosurgery in particular, several studies have shown its usefulness as a substitute for dural repair in both cranial and spine surgeries.[
Due to its similarities to the dura mater and its immunosuppressive effect, HAM has been considered a suitable substitute for dura. With this systematic review, we aim to establish the clinical applications of HAM grafts in dural repair in neurosurgery as a successful and robust dural substitute, with comparisons to already well-known dural graft materials.
MATERIALS AND METHODS
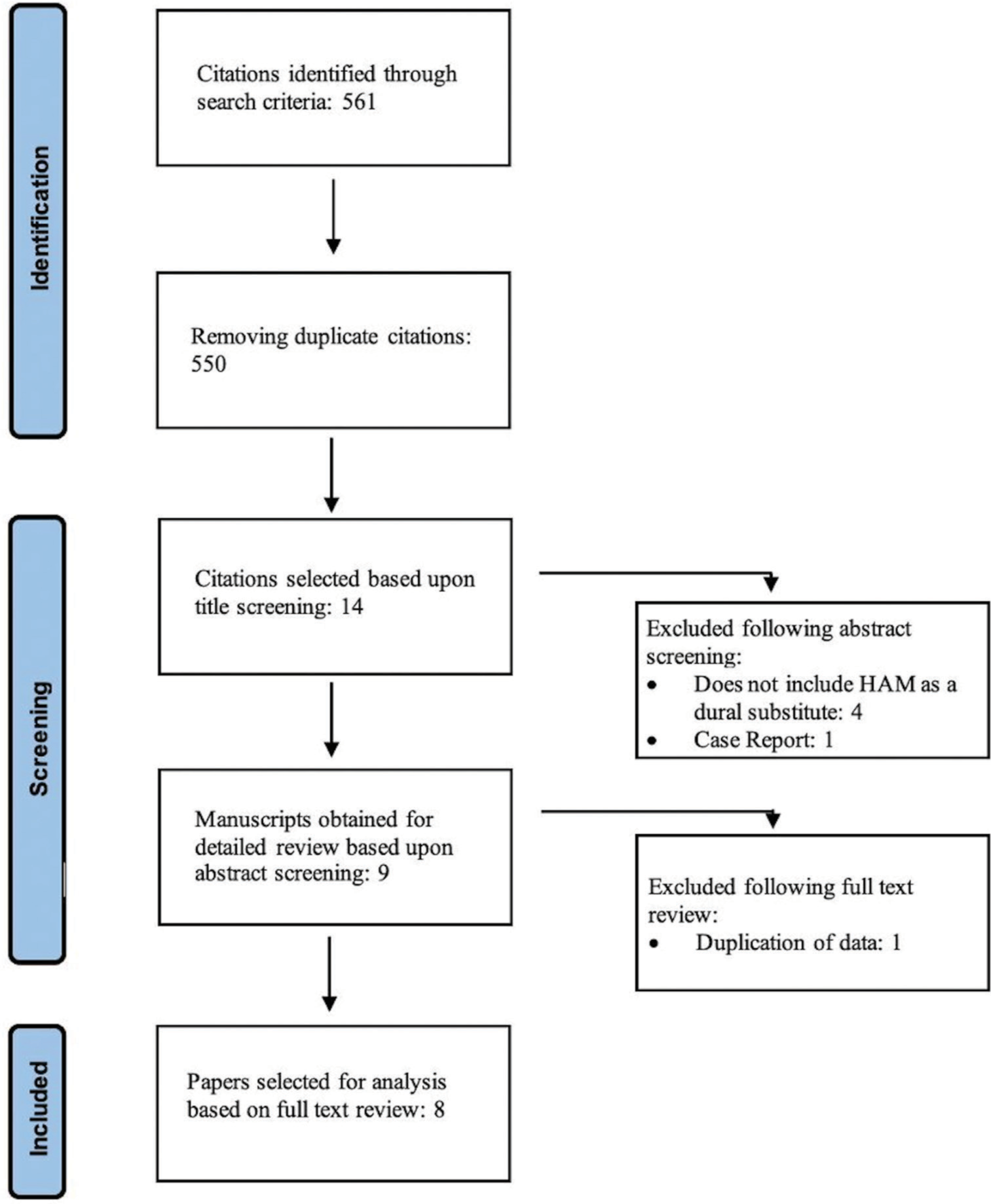
A literature search was performed using PubMed, Scopus, and Google Scholar databases after a predefined search strategy. This combined three search terms, “human amniotic membrane,” “dural repair,” and “neurosurgery,” with Boolean operators “AND” and “OR.” As a result, our search strategy was (((Human Amniotic Membrane) OR (Amniotic membrane) OR (HAM) OR (Amnion)) AND ((Dural repair) OR (Duraplasty) OR (Dural substitute)) AND ((Neurosurgery) OR (Craniectomy) OR (Craniotomy) OR (Meningioma) OR (posterior fossa tumor) OR (brain tumor) OR (spine tumor) OR (neuro-oncology) OR (Brain surgery) OR (Spine surgery))). To be eligible for inclusion in our review, papers had to report primary data, be published in English and report dural repair on humans with HAM. An initial title screen was independently done by two neurosurgical experts (Reviewer 1 [Z.S.]) and Reviewer 2 [H.B.]) followed by an independent review of abstracts. Full articles were reviewed where ambiguity regarding eligibility remained and discussed with a third arbiter (S.B.).
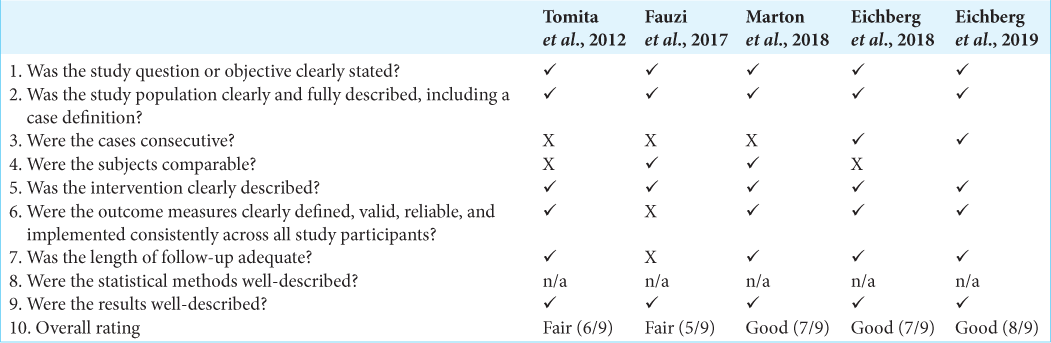
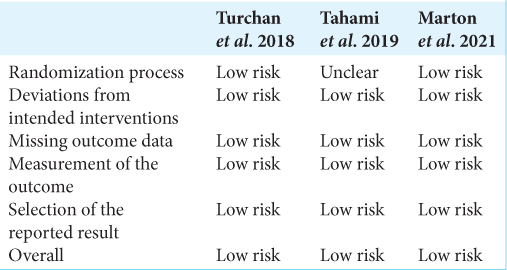
The selected articles were reviewed and data describing the authors of the study, year of publication, type of study, total number of patients, demographics of patients, presented pathology, type of neurosurgical procedure, number of patients with HAM dural repair, number of patients with synthetic and biological dural repair, and outcomes of procedure were extracted. Due to a paucity of comparative data between cohorts, meta-analysis was not possible. Qualitative assessment was conducted using the NIH Quality Assessment Tool for Case Series Studies and randomized control trials (RCTs) using the Cochrane Risk of Bias (RoB 2) assessment tool [
RESULTS
From a total of 561 articles identified from the original search algorithm, eight were identified which met all inclusion criteria for incorporation in our subjective analysis.
Risk of bias assessment
Three studies were reported as good quality studies and two reported as fair quality according to the NIH Quality Assessment Tool for Case Series Studies [
HAM as a dural substitute in trauma neurosurgery
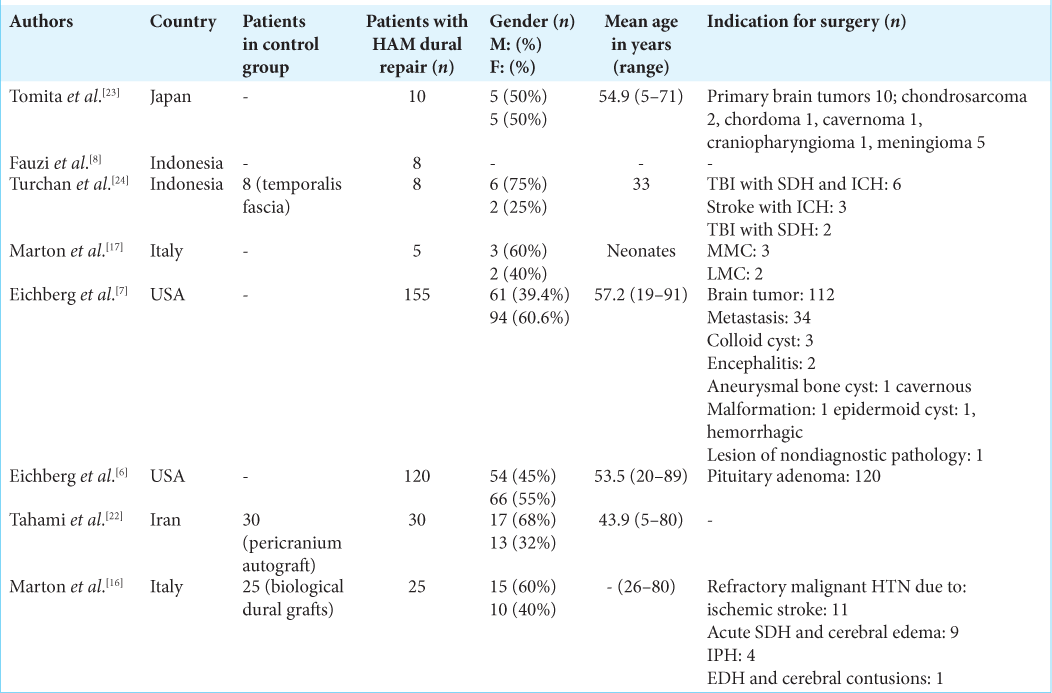
Fauzi et al. from Indonesia reported their experience of using HAM in eight patients who underwent cranioplasty after decompressive craniectomy. The article did not report demographic details of the patients nor the pathologies with which they had presented. The authors observed no cases of postoperative cerebrospinal fluid (CSF) leak in their series. Postsurgical histological analysis showed adequate fibrocyte infiltration.[
HAM as a dural substitute in elective cranial neurosurgery
Tomita et al. from Japan published the very first study reporting HAM as a dural substitute in 2012. They used HAM in a cohort of 10 patients who underwent surgery for skull base brain tumors, including meningioma (five), chondrosarcoma (two), chordoma (one), cavernoma (one), and craniopharyngioma (one). Half of the patients were male and the average age of the patients was 54.9 with their age ranging from 5 to 71 years. None of their patients developed postoperative CSF leak or experienced any other adverse reactions.[
HAM as a dural substitute in spine surgery
Marton et al. from Italy reported their experience with HAM as a dural substitute in five neonates who underwent spinal dysraphism repair including three neonates diagnosed with myelomeningocele and two diagnosed with lipomeningocele. The authors reported no postoperative complications among the neonates.[
In
DISCUSSION
In this systematic review, we report postoperative clinical outcomes associated with dural repair utilizing HAM as a dural substitute, including the frequency of CSF leak, subdural CSF collection, pseudomeningocele, hydrocephalus, meningitis, and adverse reactions reported in the current literature. We have also reported the existing literature on histological changes following dural repair using HAM as a dural substitute. Although to date, there is a limited literature on the topic with very few studies looking at long-term histological changes or long-term clinical outcomes, review of literature clearly shows HAM as a dural substitute to be comparable with clinical outcomes of dural repair using other biological or synthetic substitutes.
Several neurosurgical conditions require some form of a dural substitute to cover the exposed brain, including resection of dural-based lesions, trauma, expansile duroplasty, and posterior fossa surgeries. This step of the neurosurgical procedure helps in decreasing the incidence of CSF leak, preventing infections, and is also beneficial when performing a second surgery in avoiding cortical injury.[
Turchan et al. compared HAM with temporalis muscle fascia as a dural substitute in patients who underwent decompressive craniectomy followed by duraplasty. The author reported HAM to be comparable with temporal fascia and was able to provide watertight dural closure with no CSF leak.[
Several studies have reported on the clinical outcomes in patients in whom HAM is utilized as a dural substitute including CSF leak, CSF collection in subdural space, pseudomeningocele, hydrocephalus, meningitis, and adverse reactions.[
Limitations
The substrate population was variable in the studies included in this review and the data were nonuniformly presented, so we could not perform a meta-analysis. Of the eight studies included in this review, only three were RCTs, and the largest sample size was 60, which limits the generalizability of these results. Although we present our review on all neurosurgical procedures, only one study reports HAM utilized as a dural substitute in spine surgery. There is a need of further studies with larger sample sizes, to evaluate HAM as a dural substitute in cranial and spine surgeries to better understand the qualities of this relatively novel dural substitute.
CONCLUSION
HAM grafting can be routinely integrated into neurosurgical procedures. Studies have shown favorable results in patients undergoing dural repair in cranial and spine surgeries with HAM. Postoperative outcomes including CSF leaks, infections, and adverse reactions in patients with amniotic membrane dural repair have shown to be comparable with currently used dural materials.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alio JL, Abad M, Scorsetti DH. Preparation, indications and results of human amniotic membrane transplantation for ocular surface disorders. Expert Rev Med Devices. 2005. 2: 153-60
2. Bhosle R, Patel SS, Raju D, Ghosh N, Krishnan P. Everted pedicled temporalis fascial flap to augment pedicled pericranial flap for lax duraplasty in decompressive craniotomy: A cost-effective procedure. J Neurosci Rural Pract. 2021. 12: 438-40
3. Bohoun CA, Goto T, Morisako H, Nagahama A, Tanoue Y, Ohata K. Skull base dural repair using autologous fat as a dural substitute: An efficient technique. World Neurosurg. 2019. 127: e896-900
4. Caroli E, Rocchi G, Salvati M, Delfini R. Duraplasty: Our current experience. Surg Neurol. 2004. 61: 55-59 discussion 59
5. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011. 49: 323-8
6. Eichberg DG, Ali SC, Buttrick SS, Komotar RJ. The use of dehydrated amniotic membrane allograft for augmentation of dural closure in craniotomies and endoscopic endonasal transphenoidal surgeries. Br J Neurosurg. 2018. 32: 516-20
7. Eichberg DG, Richardson AM, Brusko GD, Ali SC, Buttrick SS, Shah AH. The use of dehydrated amniotic membrane allograft for augmentation of dural repair in transsphenoidal endoscopic endonasal resection of pituitary adenomas. Acta Neurochir (Wien). 2019. 161: 2117-22
8. Fauzi AA, Paramadini AW, Rochman TF, editors. Clinical Trial and Preparation of Amniotic Membrane as Dura Mater Artificial. 2017 5th International Conference on Instrumentation, Communications, Information Technology, and Biomedical Engineering (ICICI-BME). Bandung, Indonesia: IEEE; 2017. p. 18-20
9. Ganatra MA. Amniotic membrane in surgery. J Pak Med Assoc. 2003. 53: 29-32
10. Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea. 2006. 25: S53-8
11. Ilic D, Vicovac L, Nikolic M, Ilic E. Human amniotic membrane grafts in therapy of chronic non-healing wounds. Br Med Bull. 2016. 117: 59-67
12. Jirsova K, Jones GL. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017. 18: 193-204
13. Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001. 42: 1539-46
14. Lam FC, Penumaka A, Chen CC, Fischer EG, Kasper EM. Fibrin sealant augmentation with autologous pericranium for duraplasty after suboccipital decompression in Chiari 1 patients: A case series. Surg Neurol Int. 2013. 4: 6
15. Leal-Marin S, Kern T, Hofmann N, Pogozhykh O, Framme C, Borgel M. Human amniotic membrane: A review on tissue engineering, application, and storage. J Biomed Mater Res B Appl Biomater. 2021. 109: 1198-215
16. Marton E, Giordan E, Gallinaro P, Curzi C, Trojan D. Paolin n decompressive craniectomies. J Clin Neurosci. 2021. 89: 412-21
17. Marton E, Giordan E, Gioffre G, Canova G, Paolin A, Mazzucco MG. Homologous cryopreserved amniotic membrane in the repair of myelomeningocele: Preliminary experience. Acta Neurochir (Wien). 2018. 160: 1625-31
18. Meller D, Pauklin M, Thomasen H, Westekemper H, Steuhl KP. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011. 108: 243-8
19. Raghavan A, Wright JM, Huang Wright C, Sajatovic M, Miller J. Effect of dural substitute and technique on cranioplasty operative metrics: A systematic literature review. World Neurosurg. 2018. 119: 282-9
20. Sabatino G, Pepa GM, Bianchi F, Capone G, Rigante L, Albanese A. Autologous dural substitutes: A prospective study. Clin Neurol Neurosurg. 2014. 116: 20-3
21. Sridhar U, Tripathy K, editors. Amniotic Membrane Graft. StatPearls. Treasure Island: StatPearls; 2022. p.
22. Tahami SA, Afshar-Fereydonian N, Kazemi F, Taheri M. Comparing the results of duraplasty using amniotic membrane versus pericranium as dural graft; Concerning CSF leakage and pseudomeningocele. Br J Neurosurg. 2020. 34: 51-4
23. Tomita T, Hayashi N, Okabe M, Yoshida T, Hamada H, Endo S. New dried human amniotic membrane is useful as a substitute for dural repair after skull base surgery. J Neurol Surg B Skull Base. 2012. 73: 302-7
24. Turchan A, Rochman TF, Ibrahim A, Fauziah D, Wahyuhadi J, Parenrengi MA. Duraplasty using amniotic membrane versus temporal muscle fascia: A clinical comparative study. J Clin Neurosci. 2018. 50: 272-6