- Department of Neurological Surgery, The Ohio State University Wexner Medical Center, United States
- Department of Radiation Oncology, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio, United States
Correspondence Address:
Chi Shing Adrian Lam, Department of Neurological Surgery, The Ohio State University Wexner Medical Center, Columbus, Ohio, United States.
DOI:10.25259/SNI_53_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Chi Shing Adrian Lam1, Vicente de Paulo Martins Coelho1, Seth Wilson1, Joshua Palmer2, Anas Bardeesi1, Vikram Chakravarthy1. Hybrid therapy and use of carbon-fiber-reinforced polyetheretherketone instrumentation for management of mobile spine chordomas: A case series and review of the literature. 11-Apr-2025;16:130
How to cite this URL: Chi Shing Adrian Lam1, Vicente de Paulo Martins Coelho1, Seth Wilson1, Joshua Palmer2, Anas Bardeesi1, Vikram Chakravarthy1. Hybrid therapy and use of carbon-fiber-reinforced polyetheretherketone instrumentation for management of mobile spine chordomas: A case series and review of the literature. 11-Apr-2025;16:130. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13497
Abstract
BackgroundMobile spine chordomas demonstrate varied surgical risk profiles compared to their sacral analogs. Often, the limitation to performing an en bloc resection of a mobile spine chordoma is tumor violation of the epidural space. Given these limitations, we propose the utilization of carbon fiber-reinforced polyetheretherketone (CFR-PEEK) instrumentation in separation surgery to enhance visualization for stereotactic body radiation therapy (SBRT) planning, allowing an ablative radiosurgical dose to be delivered.
MethodsWe present two illustrative cases highlighting the advantages of hybrid therapy (separation surgery and adjuvant SBRT) with CFR-PEEK instrumentation in the management of mobile spine chordoma.
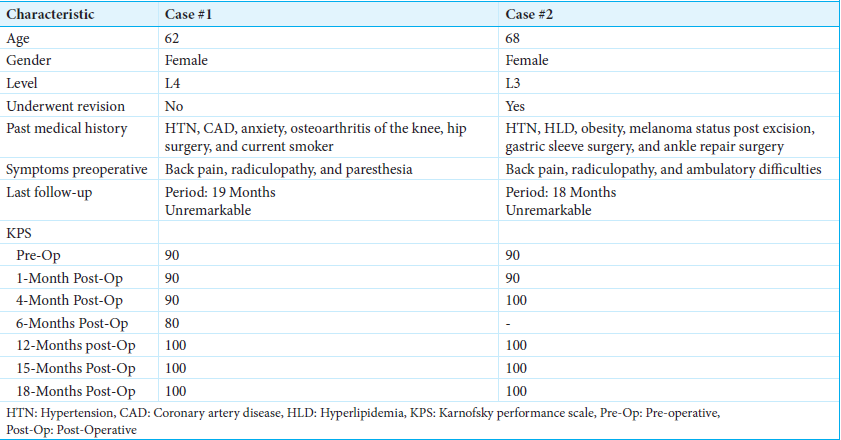
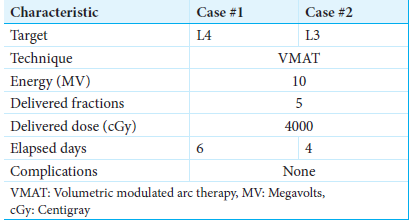
ResultsCase 1 is a 62-year-old female with an L4 chordoma who underwent separation surgery and L3–5 posterior instrumented fusion using CFR-PEEK instrumentation. Case 2 is a 68-year-old female with a L3 chordoma who underwent revision separation surgery encompassing completion of L3 partial corpectomy and CFR-PEEK screw exchange of prior L2–4 titanium instrumentation. Both patients received postoperative ablative SBRT. At 18-month postoperative time points, both patients were clinically stable, with no signs of tumor recurrence or progression.
ConclusionMobile spine chordomas present a unique challenge in obtaining a margin negative en bloc resection. Separation surgery allows the ability to decrease surgical morbidity and deliver an ablative radiosurgical dose. Furthermore, the incorporation of CFR-PEEK instrumentation allows the utilization of multiparametric magnetic resonance imaging for long-term disease monitoring. Hybrid therapy, a less morbid alternative to standard en bloc spondylectomy, offers a better surgical morbidity profile by combining effectively with SBRT for optimal tumor control.
Keywords: Carbon fiber/polyetheretherketone, Chordoma, Hybrid therapy, Magnetic resonance imaging, Mobile spine, Separation surgery
INTRODUCTION
Chordomas of the mobile spine (C1-L5) are rare primary osseous neoplasms with an annual incidence of 0.05/100,000 people per year.[
According to the Enneking staging system, chordomas are classified as low-grade malignant tumors, which fall under stages IA and IB.[
Shifting from therapeutic approaches, radiographic imaging to evaluate chordomas typically involves computed tomography (CT) scans and magnetic resonance imaging (MRI). On CT, these tumors have low density and may have cortical destruction.[
Innovations in surgical technology can assist in the multi-disciplinary nature of standard care for chordoma patients. Titanium-based surgical constructs produce artifacts on follow-up CT and MRI, creating challenges in radiation planning and oncologic surveillance.[
We report two cases of chordoma in the mobile spine where CFR-PEEK implants were utilized in separation surgery followed by adjuvant SBRT, reporting patient outcomes and benefits of this combined treatment modality.
MATERIALS AND METHODS
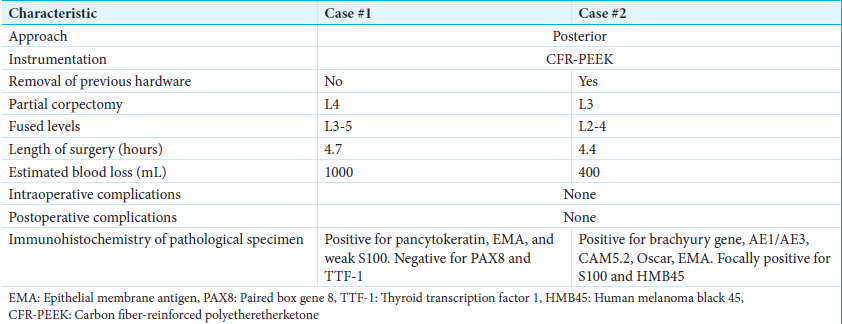
We describe two cases of mobile spine chordomas [
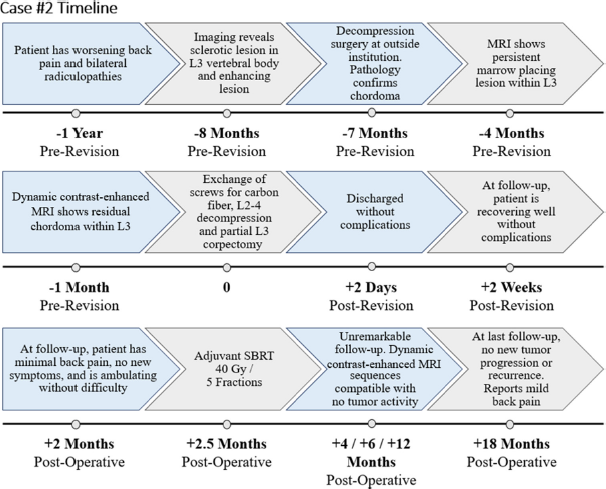
Case 2 features a 68-year-old female presenting with back pain and bilateral lower extremity radiculopathy impacting ambulation. The patient underwent partial resection of L3 epidural tumor at an outside hospital, with vertebroplasty at the index level and titanium pedicle screw fixation from L2 to 4. Pathology confirmed chordoma and radiation was not performed. Six-month postoperative imaging revealed a residual tumor. The patient then underwent revision surgery with transpedicular L3 partial corpectomy and L2–4 pedicle screw exchange with CFR-PEEK to assist with postoperative radiation therapy planning.
Patient informed consent was obtained, and this case series has been reported in line with the CARE guidelines (for case reports).[
RESULTS
Case 1
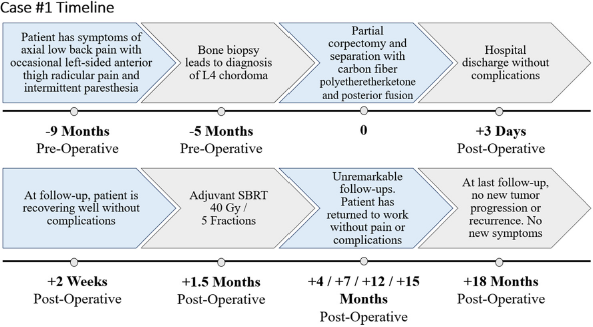
A 62-year-old female presented with a 4-month history of axial low back pain with occasional left-sided anterior thigh radicular pain and intermittent paresthesia [
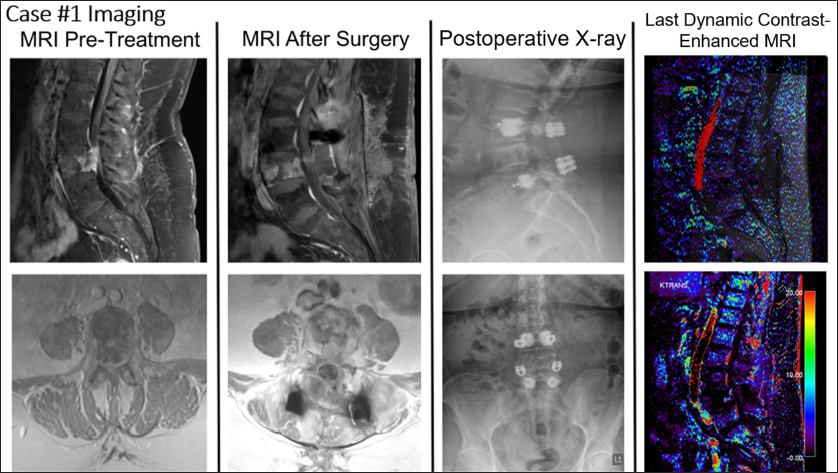
Imaging revealed a T2 hyperintense destructive expansile lesion at L4, demonstrating cortical breakthrough into the anterior epidural space and anterior left prevertebral soft tissues [
Figure 2:
Relevant imaging for Case #1. The first column contains T1-weighted fat-suppressed magnetic resonance imaging (MRI) pre-treatment (top = sagittal, bottom = axial). The second column contains T1-weighted fat-suppressed MRI after surgery (top = sagittal, bottom = axial). The third column contains X-rays after surgery (top = lateral, bottom = anteroposterior). The last column contains the last dynamic contrast-enhanced-MRI (top = overlay, bottom = MR perfusion).
Biopsy of the L4 vertebrae demonstrated chordoma consistent with the conventional subtype. Immunohistochemistry confirmed tumor cells positive for pancytokeratin, epithelial membrane antigen (EMA), and weak S100; paired box gene 8 and thyroid transcription factor 1 stain were negative. Expression of p53 was low, where 20% of tumor cell nuclei had intense p53 immunoreactivity, associated with improved prognosis and longer progression-free survival in chordomas.[
Thereafter, she underwent separation surgery, encompassing a partial transpedicular corpectomy of L4 with L3–5 cement-augmented posterior instrumented fusion, using CFR-PEEK instrumentation [
At 1-month postoperative, imaging demonstrated adequate neural decompression. Thereafter, she underwent SBRT using 3 volumetric modulated arc therapy (VMAT) at 40 Gy in five fractions with 10 megavolts (MV) photons in a flattening filter-free (FFF) mode [
Figure 3:
Dosimetry mapping for Case #1 (left) and Case #2 (right). This figure highlights typical volumetric modulated arc therapy spinal stereotactic body radiation therapy isocenter planning used in these cases, following consensus contouring guidelines. Of note, no hardware-related imaging artifact at the target volume allowed precise dose delineation.
Three- and 6-month MRIs were stable, and the patient reported a Karnofsky Performance Scale (KPS) of 100 at 19 months postoperative. Dynamic contrast-enhanced MRI (DCE-MRI) scans were performed postoperatively at 3 weeks, 5 weeks, 4 months, 7 months, 10 months, 13, and 19 months to evaluate for progression. DCE-MRI revealed stable findings consistent with postoperative changes and no evidence of tumor activity or recurrence.
Case 2
A 68-year-old female presented with worsening back pain with radiation into bilateral lower extremities [
Figure 5:
Relevant imaging for Case #2. The first column contains T1-weighted fat-suppressed magnetic resonance imaging (MRI) pre-treatment (top = sagittal, bottom = axial). The second column contains T1-weighted fat-suppressed MRI after her first surgery (top = sagittal, bottom = axial). The third column contains T1-weighted fat-suppressed MRI after her revision surgery (top = sagittal, bottom = axial). The fourth column contains sagittal X-rays before (top) and after (bottom) revision surgery. The last column contains the last dynamic contrast-enhanced-MRI (top = overlay, bottom = MR perfusion).
She underwent laminectomy and partial facetectomies of L3 bilaterally, debulking of L3 epidural tumor, L3 vertebroplasty, and L2–4 pedicle screw fixation with titanium instrumentation at an outside institution. Pathology confirmed the diagnosis of chordoma, with tumor cells positive for multiple cytokeratins and the brachyury gene. Cytokeratins AE1/AE3, CAM5.2, and Oscar were found to be diffusely positive in tumor cells. EMA stained positive, while S100 and human melanoma black 45 were focally positive.
At 3 months postoperative, an MRI lumbar spine demonstrated a persistent marrow placing lesion. At 6 months postoperative, she presented to our institution for evaluation. MRI lumbar spine revealed residual chordoma within the L3 vertebral body with increased right prevertebral extraosseous extension and bilateral L3–4 foraminal tumoral compression. The patient also experienced mechanical back pain, and a CT scan demonstrated loose hardware. On physical examination, she was neurologically intact, with 5/5 motor strength in all extremities.
Before the revision, KPS was 90. Revision surgery consisted of re-do decompression/epidural tumor debulking with L3 partial corpectomy and L3–5 instrumentation replacement with CFR-PEEK [
The patient then underwent postoperative SBRT, consisting of a cumulative dose of 40 Gy in 5 fractions using 2 VMAT arcs with 10 MV photons in an FFF mode targeting the L3 vertebra [
DISCUSSION
Overview of the management of mobile spine chordomas
Mobile spine chordomas demonstrate a slow, indolent growth rate, often diagnosed at later stages, with patients remaining asymptomatic for a lengthy period.[
Conventionally, chordomas have been thought of as radio- and chemo-resistant tumors that require aggressive surgical control.[
At present, gaps remain in the literature and consensus does not exist regarding the optimal strategy to manage patients with mobile spine chordomas. Debates remain in balancing aggressive surgical resections at the risk of sacrificing neurological function. In a retrospective study of 26 mobile spine chordoma patients who underwent surgical resection, 73% experienced complications, such as deep infection and neurological complications, with 35% requiring reoperation.[
Meanwhile, in a multi-institutional study of 32 patients with mobile spine chordomas, Molina et al.[
Furthermore, en bloc resection does not guarantee an R0 resection, and even if an R0 resection is achieved, there could still be microscopic residual disease. Over the years, studies have increasingly highlighted the importance of neoadjuvant or adjuvant high-dose radiotherapy for the management of spinal chordomas.[
Further studies have suggested coupling intralesional, gross-total resections or separation surgery with postoperative SBRT can be an alternative technique to en bloc resections. In a single-institution study of 12 patients with mobile spine chordomas, Lockney et al.[
In a single institution study where 16 chordoma patients were treated with varying approaches, Akmansu et al.[
CFR-PEEK benefits over titanium for posttreatment monitoring and radiation effectiveness
A key principle in radiation planning is to maximize the tumoricidal dose, or the biologically effective dose while minimizing damage to the surrounding tissues. Having clear visualization for radiation therapy planning is key for radiation oncology treatments, staging scans, and dosimetry protocols.
As spine surgeons have transitioned away from using traditional autografts, the prevalence of interbody device materials has grown.[
Compared to traditional titanium implants, CFR-PEEK instrumentation provides a radiolucent biomaterial with similar clinical properties to the native spine.[
Out of 1400 patients treated by the National Centre for Oncological Hadron Therapy with proton and carbon therapy, Mastella et al.[
In a retrospective study of ten patients with spinal metastases who underwent postoperative photon therapy, Müller et al.[
In addition to enhanced visualization for adjuvant treatment, the use of CFR-PEEK instrumentation has demonstrated improved efficacy monitoring for local disease recurrence.[
While beneficial for all primary and metastatic spine diseases, this may play a significant role in the management of chordoma due to high reported rates of local recurrence.[
Dosimetry for enhanced SBRT efficacy in chordoma treatment
Proper dosing of adjuvant radiation therapy is key in chordoma management. Jin et al.[
In a study completed by Chen et al.,[
The tumor that is located too close to the spinal cord may indicate the need for proton therapy, as dosing to the spinal cord is one of the main complications of SBRT.[
Although studies have found proton therapy to be advantageous to photon therapy due to the depth-dose characteristics of protons that allow for better sparing of normal tissues,[
Yazici et al.[
Multiparametric post-treatment monitoring
Radiographic imaging for evaluating chordomas typically involves CT and MRI. On CT, these tumors have low density and may have cortical destruction.[
Santos et al.[
CFR-PEEK also allows for better disease monitoring. Titanium implants can cause artifacts, leading to an inability to use multiparametric studies reliably. These have shown MRI to be significantly affected by titanium materials.[
Limitations
Optimal management approaches for spine chordomas are debated. Although surgical resection is a mainstay, it is more challenging to obtain negative margin resection in mobile spine chordomas, given the anatomic constraints.
The present study is limited by its patient volume and longitudinal follow-up; further work will be required to determine whether CFR-PEEK hardware significantly affects the morbidity and mortality of patients with mobile spinal chordomas. Although a small series, early follow-up demonstrates a lack of metabolic activity supporting ablative treatment of the chordoma. Ongoing surveillance with DCE-MRI will confirm absence of active chordoma disease. While many pros of CFR-PEEK instrumentation have been highlighted, other factors for consideration include the cost comparison of titanium versus CFR-PEEK implants.
CONCLUSION
Inherent anatomic differences between the sacrum and mobile spine lead to consequently distinct morbidity outlooks for the traditional en bloc resection dogma. This scenario propels separation surgery as an appealing surgical strategy for this location. With advances in SBRT and the ability to deliver an ablative dose, inducing tumor apoptosis/ necrosis, shifting prior paradigms of radiosensitivity. Hybrid therapy, integrating separation surgery and adjuvant SBRT, has recently demonstrated success in managing chordomas. This integrated approach may be a noteworthy advancement in the comprehensive care of mobile spine chordomas.
Ethical approval
The Institutional Review Board approval is obtained from the Ohio State University and approval number is: 2022C0198 dated 09/07/2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We would like to thank the patients highlighted in this series whose cases have benefitted neurosurgical research and education.
References
1. Akmansu M, Kurt G, Demircan V, Senturk E. Results of chordoma patients treated by different approaches in a single institution. Turk Neurosurg. 2020. 30: 366-70
2. Almefty K, Pravdenkova S, Colli BO, Al-Mefty O, Gokden M. Chordoma and chondrosarcoma: Similar, but quite different, skull base tumors. Cancer. 2007. 110: 2467-77
3. Barzilai O, Amato MK, McLaughlin L, Reiner AS, Ogilvie SQ, Lis E. Hybrid surgery-radiosurgery therapy for metastatic epidural spinal cord compression: A prospective evaluation using patient-reported outcomes. Neurooncol Pract. 2018. 5: 104-13
4. Boriani S, Bandiera S, Biagini R, Bacchini P, Boriani L, Cappuccio M. Chordoma of the mobile spine: Fifty years of experience. Spine (Phila Pa 1976). 2006. 31: 493-503
5. Boriani S. En bloc resection in the spine: A procedure of surgical oncology. J Spine Surg. 2018. 4: 668-76
6. Chen X, Lo SF, Bettegowda C, Ryan DM, Gross JM, Hu C. High-dose hypofractionated stereotactic body radiotherapy for spinal chordoma. J Neurosurg Spine. 2021. 35: 674-83
7. Cofano F, Di Perna G, Monticelli M, Marengo N, Ajello M, Mammi M. Carbon fiber reinforced vs titanium implants for fixation in spinal metastases: A comparative clinical study about safety and effectiveness of the new “carbon-strategy. ” J Clin Neurosci. 2020. 75: 106-11
8. Court C, Briand S, Mir O, Péchoux CL, Lazure T, Missenard G. Management of chordoma of the sacrum and mobile spine. Orthop Traumatol Surg Res. 2022. 108: 103169
9. Delank KS, Kriegsmann J, Drees P, Eckardt A, Eysel P. Metastasizing chordoma of the lumbar spine. Eur Spine J. 2002. 11: 167-71
10. Di Perna G, Cofano F, Mantovani C, Badellino S, Marengo N, Ajello M. Separation surgery for metastatic epidural spinal cord compression: A qualitative review. J Bone Oncol. 2020. 25: 100320
11. Erdem E, Angtuaco EC, Van Hemert R, Park JS, Al-Mefty O. Comprehensive review of intracranial chordoma. Radiographics. 2003. 23: 995-1009
12. Geibel MA, Gelißen B, Bracher AK, Rasche V. Artifact properties of dental ceramic and titanium implants in MRI. Rofo. 2019. 191: 433-41
13. Hanna SA, Aston WJ, Briggs TW, Cannon SR, Saifuddin A. Sacral chordoma: Can local recurrence after sacrectomy be predicted?. Clin Orthop Relat Res. 2008. 466: 2217-23
14. Herzog LN, Traven SA, Walton ZJ, Leddy LR. The use of carbon fiber implants for impending or existing pathologic fractures. J Orthop Trauma. 2022. 36: e260
15. Hwang S, Hameed M, Kransdorf M. The 2020 World Health Organization classification of bone tumors: What radiologists should know. Skeletal Radiol. 2023. 52: 329-48
16. Jawad MU, Scully SP. In brief: Classifications in brief: Enneking classification: Benign and malignant tumors of the musculoskeletal system. Clin Orthop Relat Res. 2010. 468: 2000-2
17. Jin CJ, Berry-Candelario J, Reiner AS, Laufer I, Higginson DS, Schmitt AM. Long-term outcomes of high-dose single-fraction radiosurgery for chordomas of the spine and sacrum. J Neurosurg Spine. 2019. 32: 79-88
18. Joerger AK, Seitz S, Lange N, Aftahy AK, Wagner A, Ryang YM. CFR-PEEK Pedicle screw instrumentation for spinal neoplasms: A single center experience on safety and efficacy. Cancers (Basel). 2022. 14: 5275
19. Jung EW, Jung DL, Balagamwala EH, Angelov L, Suh JH, Djemil T. Single-fraction spine stereotactic body radiation therapy for the treatment of chordoma. Technol Cancer Res Treat. 2017. 16: 302-9
20. Kolz JM, Wellings EP, Houdek MT, Clarke MJ, Yaszemski MJ, Rose PS. Surgical treatment of primary mobile spine chordoma. J Surg Oncol. 2021. 123: 1284-91
21. Konieczkowski DJ, DeLaney TF, Yamada YJ. Radiation strategies for spine chordoma: Proton beam, carbon ions, and stereotactic body radiation therapy. Neurosurg Clin N Am. 2020. 31: 263-88
22. Krätzig T, Mende KC, Mohme M, Kniep H, Dreimann M, Stangenberg M. Carbon fiber-reinforced PEEK versus titanium implants: An in vitro comparison of susceptibility artifacts in CT and MR imaging. Neurosurg Rev. 2021. 44: 2163-70
23. Kumar N, Ramakrishnan SA, Lopez KG, Madhu S, Ramos MR, Fuh JY. Can polyether ether ketone dethrone titanium as the choice implant material for metastatic spine tumor surgery?. World Neurosurg. 2021. 148: 94-109
24. Lockney DT, Shub T, Hopkins B, Lockney NA, Moussazadeh N, Lis E. Spinal stereotactic body radiotherapy following intralesional curettage with separation surgery for initial or salvage chordoma treatment. Neurosurg Focus. 2017. 42: E4
25. Mastella E, Molinelli S, Magro G, Mirandola A, Russo S, Vai A. Dosimetric characterization of carbon fiber stabilization devices for post-operative particle therapy. Phys Med. 2017. 44: 18-25
26. McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: Incidence and survival patterns in the United States 1973–1995. Cancer Causes Control. 2001. 12: 1-11
27. Meyer JE, Lepke RA, Lindfors KK, Pagani JJ, Hirschy JC, Hayman LA. Chordomas: their CT appearance in the cervical, thoracic and lumbar spine. Radiology. 1984. 153: 693-6
28. Mohan R, Grosshans D. Proton therapy-Present and future. Adv Drug Deliv Rev. 2017. 109: 26-44
29. Molina CA, Ames CP, Chou D, Rhines LD, Hsieh PC, Zadnik PL. Outcomes following attempted en bloc resection of cervical chordomas in the C-1 and C-2 region versus the subaxial region: A multiinstitutional experience. J Neurosurg Spine. 2014. 21: 348-56
30. Moussazadeh N, Laufer I, Yamada Y, Bilsky MH. Separation surgery for spinal metastases: Effect of spinal radiosurgery on surgical treatment goals. Cancer Control. 2014. 21: 168-74
31. Müller BS, Ryang YM, Oechsner M, Düsberg M, Meyer B, Combs SE. The dosimetric impact of stabilizing spinal implants in radiotherapy treatment planning with protons and photons: Standard titanium alloy vs. radiolucent carbon-fiber-reinforced PEEK systems. J Appl Clin Med Phys. 2020. 21: 6-14
32. Muthiah N, Yolcu YU, Alan N, Agarwal N, Hamilton DK, Ozpinar A. Evolution of polyetheretherketone (PEEK) and titanium interbody devices for spinal procedures: A comprehensive review of the literature. Eur Spine J. 2022. 31: 2547-56
33. Ozturk AK, Gokaslan ZL, Wolinsky JP. Surgical treatment of sarcomas of the spine. Curr Treat Options Oncol. 2014. 15: 482-92
34. Pennington Z, Ehresman J, McCarthy EF, Ahmed AK, Pittman PD, Lubelski D. Chordoma of the sacrum and mobile spine: A narrative review. Spine J. 2021. 21: 500-17
35. Redmond KJ, Lo SS, Fisher C, Sahgal A. Postoperative stereotactic body radiation therapy (SBRT) for spine metastases: A critical review to guide practice. Int J Radiat Oncol Biol Phys. 2016. 95: 1414-28
36. Riley DS, Barber MS, Kienle GS, Aronson JK, von SchoenAngerere T, Tugwellf P. CARE guidelines for case reports: Explanation and elaboration document. J Clin Epidemiol. 2017. 89: 218-35
37. Santos P, Peck KK, Arevalo-Perez J, Karimi S, Lis E, Yamada Y. T1-Weighted dynamic contrast-enhanced MR perfusion imaging characterizes tumor response to radiation therapy in chordoma. AJNR Am J Neuroradiol. 2017. 38: 2210-6
38. Shen FH, Gasbarrini A, Lui DF, Reynolds J, Capua J, Boriani S. Integrated custom composite polyetheretherketone/carbon fiber (PEEK/CF) vertebral body replacement (VBR) in the treatment of bone tumors of the spine: A preliminary report from a multicenter study. Spine. 2022. 47: 252
39. Shi C, Lin H, Huang S, Xiong W, Hu L, Choi I. Comprehensive evaluation of carbon-fiber-reinforced polyetheretherketone (CFR-PEEK) spinal hardware for proton and photon planning. Technol Cancer Res Treat. 2022. 21: 15330338221091700
40. Spratt DE, Beeler WH, de Moraes FY, Rhines LD, Gemmete JJ, Chaudhary N. An integrated multidisciplinary algorithm for the management of spinal metastases: An International Spine Oncology Consortium report. Lancet Oncol. 2017. 18: e720-30
41. Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010. 95: 3-22
42. Takayanagi A, Siddiqi I, Ghanchi H, Lischalk J, Vrionis F, Ratliff J. Radiolucent carbon fiber-reinforced implants for treatment of spinal tumors-clinical, radiographic, and dosimetric considerations. World Neurosurg. 2021. 152: 61-70
43. Tauziéde-Espariat A, Bresson D, Polivka M, Bouazza S, Labrousse F, Aronica E. Prognostic and therapeutic markers in chordomas: A study of 287 tumors. J Neuropathol Exp Neurol. 2016. 75: 111-20
44. Thureau S, Marchesi V, Vieillard MH, Perrier L, Lisbona A, Leheurteur M. Efficacy of extracranial stereotactic body radiation therapy (SBRT) added to standard treatment in patients with solid tumors (breast, prostate and non-small cell lung cancer) with up to 3 bone-only metastases: Study protocol for a randomised phase III trial (STEREO-OS). BMC Cancer. 2021. 21: 117
45. Tian K, Wang L, Ma J, Wang K, Li D, Du J. MR Imaging grading system for skull base chordoma. AJNR Am J Neuroradiol. 2017. 38: 1206-11
46. Ulici V, Hart J. A Review and differential diagnosis. Arch Pathol Lab Med. 2022. 146: 386-95
47. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012. 13: e69-76
48. Ward J, Damante M, Wilson S, Coelho V, Franceschelli D, Elguindy AN. Impact of instrumentation material on local recurrence: A case-matched series using carbon fiber-PEEK vs. titanium. J Neurooncol. 2025. 171: 155-62
49. Ward J, Damante M, Wilson S, Elguindy AN, Franceschelli D, Coelho Junior VP. Use of magnetic resonance imaging for postoperative radiation therapy planning in patients with carbon fiber-reinforced polyetheretherketone instrumentation. Pract Radiat Oncol. 2024. p. ???:S1879-8500(24)00296-0
50. Ward J, Wilson S, Eaton RG, Coelho V, Xu DS, Chakravarthy VB. Emerging role of carbon fiber-reinforced polyetheretherketone instrumentation in spinal oncology: A systematic review. Adv Radiother Nucl Med. 2024. 2: 3130
51. World Health Organization. Soft tissue and bone tumours. Available from: https://publications.iarc.fr/book-and-report-series/who-classification-of-tumours/soft-tissue-and-bone-tumours-2020 [Last accessed on 2023 Dec 27].
52. Yamada Y, Gounder M, Laufer I. Multidisciplinary management of recurrent chordomas. Curr Treat Options Oncol. 2013. 14: 442-53
53. Yazici G, Sari SY, Yedekci FY, Yucekul A, Birgi SD, Demirkiran G. The dosimetric impact of implants on the spinal cord dose during stereotactic body radiotherapy. Radiat Oncol. 2016. 11: 71
54. York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999. 44: 74-79 discussion 79-80
55. Zuckerman SL, Bilsky MH, Laufer I. Chordomas of the skull base, mobile spine, and sacrum: An epidemiologic investigation of presentation, treatment, and survival. World Neurosurg. 2018. 113: e618-27