- Department of Neurosurgery, Centro Hospitalar Universitario Lisboa Central, R. Jose Antonio Serrano, Lisbon, Lisbon, Portugal.
- Department of Pathological Anatomy, Centro Hospitalar Universitario Lisboa Central, R. Jose Antonio Serrano, Lisbon, Lisbon, Portugal.
Correspondence Address:
Ricardo Malcata Nogueira
Department of Pathological Anatomy, Centro Hospitalar Universitario Lisboa Central, R. Jose Antonio Serrano, Lisbon, Lisbon, Portugal.
DOI:10.25259/SNI_231_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ricardo Malcata Nogueira1, Luis Santos Cardoso1, Lino Fonseca1, Miguel Correia1, Amets Iraneta1, Pedro Roque1, Mario Matos1, Manuela Mafra2. Hydrocephalus in children – A rare case of pineal cavernoma and literature review. Surg Neurol Int 18-Sep-2020;11:294
How to cite this URL: Ricardo Malcata Nogueira1, Luis Santos Cardoso1, Lino Fonseca1, Miguel Correia1, Amets Iraneta1, Pedro Roque1, Mario Matos1, Manuela Mafra2. Hydrocephalus in children – A rare case of pineal cavernoma and literature review. Surg Neurol Int 18-Sep-2020;11:294. Available from: https://surgicalneurologyint.com/surgicalint-articles/10268/
Abstract
Background: Cavernous malformations prevalence ranges from 0.4 to 0.6% and accounts for 5–15% of all central nervous system vascular malformations. Pineal cavernomas constitute <1% of all locations published in the literature, with a total of 26 cases reported, only 5 regarding the pediatric population until 2020. Overall annual hemorrhage rate is 2.4%. Symptoms are often due to hydrocephalus and intracranial hypertension.
Case Description: We report a case of a 5-year-old child with visual disturbances, headache, and progressive neurologic deterioration. MR showed a lesion in the pineal region and triventricular hydrocephalus. She was submitted to endoscopic third ventriculostomy and total excision of the lesion by the infratentorial supracerebellar approach a few days later. Histopathological examination confirmed a pineal cavernous malformation. The patient returned to her normal life without any neurologic deficit and a normal development.
Conclusion: The ideal treatment is primary lesion removal; however, due to the infrequency and because it is a curable lesion, studies seeking to deepen the knowledge of this disease are considered relevant.
Keywords: Cavernous malformation, Hydrocephalus, Pediatric, Pineal, Vascular disorders
INTRODUCTION
Cavernous malformations (CMs) prevalence ranges from 0.4% to 0.6%[
Location in the pineal region is particularly uncommon,[
The literature about the natural history of CMs in the pediatric population is limited, but the development of CMs appears to increase with age, reaching a plateau in late adolescence.[
Pineal cavernomas are even more rare in the pediatric population – the youngest patient was 4 weeks old[
Cavernous malformations are clusters of dilated sinusoidal channels lined by a single layer of endothelium and lack of smooth muscle or normal brain tissue, thus classified as angiographically occult.[
The etiology of these malformations is widely discussed, but there is evidence of autosomal dominant inheritance located on chromosome 7. Radiotherapy is a known risk factor.[
The symptoms are often due to hydrocephalus and intracranial hypertension.[
CASE REPORT
We report a case of a 5-year-old child sent from an African country who presented visual disturbances and headache with a few months of development, progressive deterioration, and abrupt onset of vomiting and irritability.
The child was evacuated to Portugal with a CT-scan that showed a triventricular hydrocephalus, an MR being immediately performed. This examination showed a lesion in the pineal region and triventricular hydrocephalus, as described in
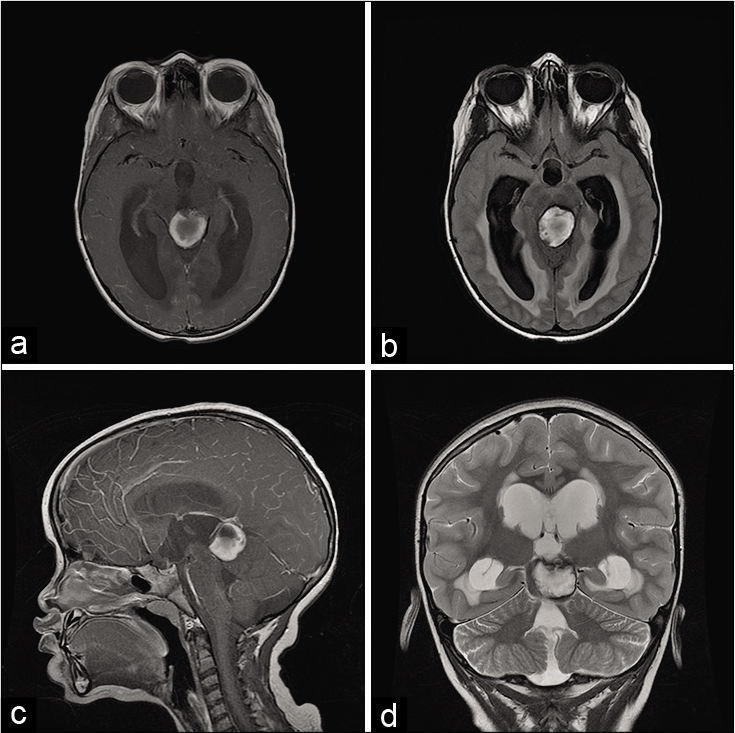
Figure 1:
(a) Preoperative axial T1 ponderation – bleeding centered in the region of quadrigeminal lamina/pineal region associated with small round heterogeneous on his left anterior aspect with triventricular hydrocephalus, (b) axial FLAIR showing active hydrocephalus with ependimary transudation, (c) sagittal T1 gadolinium showing pineal lesion with Sylvius aqueduct obstruction, (d) T2 coronal showing pineal lesion with consequent hydrocephalus, the core of the lesion is surrounded by a halo of lower intensity due to hemosiderin.
The patient was immediately submitted to endoscopic third ventriculostomy with a resolution of hydrocephalus. She later underwent suboccipital craniotomy and total excision of the lesion by the infratentorial supracerebellar approach a few days after [
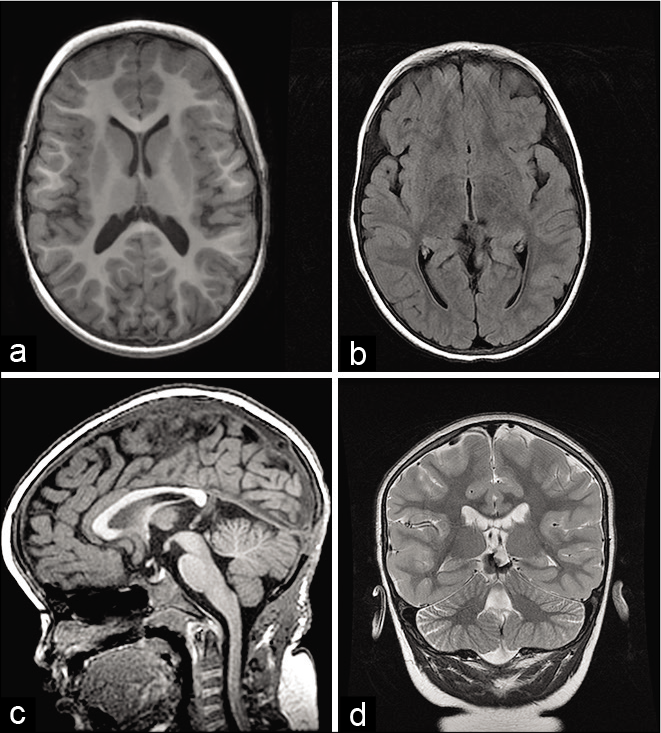
Figure 2:
(a) Axial T1 2 months after endoscopic ventriculostomy and total excision of the lesion of the pineal region, (b) axial FLAIR showing nondilated temporal horns, (c) T1 sagittal showing repermeability of Sylvius aqueduct, and (d) T2 coronal showing a small blood residue, no mass effect and without hydrocephalus signals.
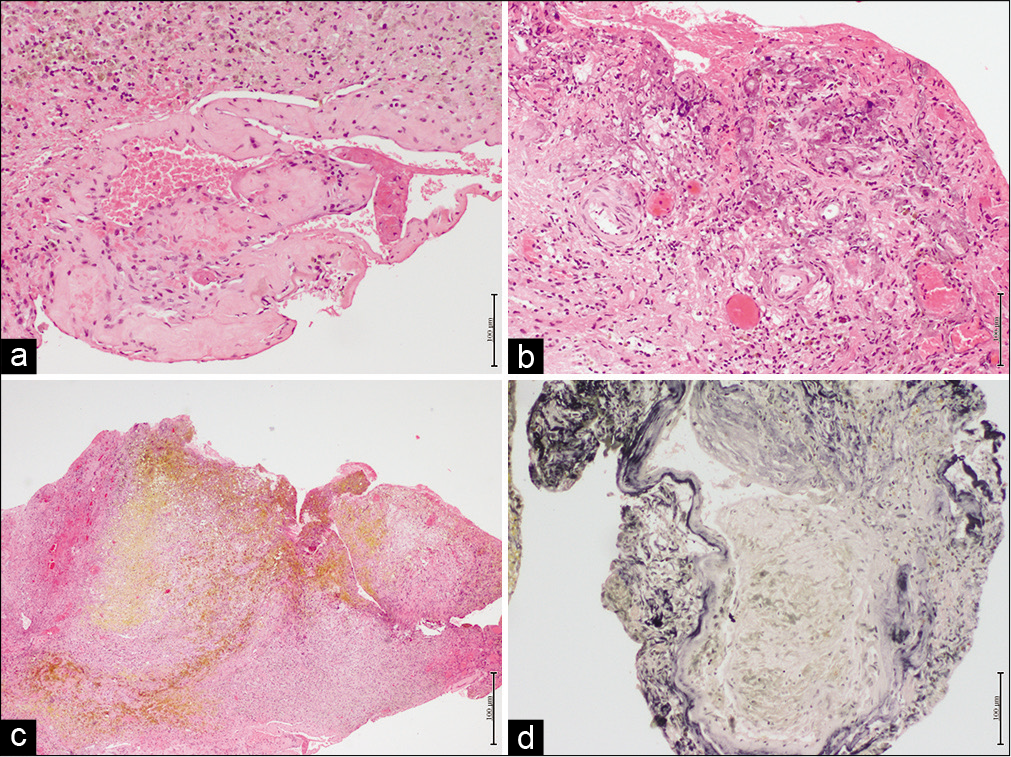
Glial tissue fragments with recent hemorrhage, dilated sinusoidal channels, and infiltration by histiocytes containing hemosiderin confirmed the suspicious diagnosis considering the MR characteristic of pineal cavernous malformation [
Figure 3:
(a) Hematoxylin-eosin shows an agglomerate of vessels of different sizes and thin walls, (b) hematoxylin-eosin shows vessels surrounded blood and histiocytic infiltrate with hemosiderin pigment, (c) hematoxylin-eosin with signs of old hemorrhage, and (d) Verhoeff coloration showing elastic fibers in black.
DISCUSSION
Symptoms and clinical approaches to cavernous malformations depend on lesion characteristics and location. Supratentorial lesions most commonly present seizures, headache, or focal neurological deficits; however, many patients are asymptomatic.[
Of the total cavernous malformations, 9–35% occur in the brainstem,[
Since pineal cavernous malformations were difficult to diagnose before the introduction of MR, some incidences of this disorder were erroneously treated with irradiation, before surgical resection was performed.[
Cavernomas are best appreciated on T2-weighted spin-echo MR, demonstrating a classic signature of “popcorn” or “berry” appearance, surrounded by a ring of low signal intensity due to hemosiderin.[
Cavernous angioma located in the pineal gland is usually accompanied by supratentorial hydrocephalus, with dilation of lateral and third ventricles, the fourth ventricle being preserved. About 75% of patients had partial or complete Parinaud’s syndrome after treatment of pineal region lesions.[
In our case, the patient presented hydrocephalus and hemorrhage in the pineal region, tumors being the most common cause for the latter.[
These malformations are very rare in the pediatric population – the youngest patient being 4 weeks old,[
The clinical manifestations related to the disease vary; a common manifestation is the sign of Parinaud, characterized mainly by difficulty in eye movement, superiorly; others may be the signs of intracranial hypertension, headache, visual disturbances, gait ataxia, and changes in circadian rhythm. In addition to these, there are still signs of hemiparesis, hemi-hypoesthesia, diabetes insipidus, and amenorrhea, with high prolactin, neuroendocrine disorders occurring mainly due to damage to the hypothalamus region by distention of the third ventricle floor.[
Recent studies[
Direct microsurgical excision of the vascular malformation is the standard treatment and when completely removed, the risk of further growth or hemorrhage of cavernous malformations is very low,[
The approaches that are generally used to access the pineal region are supracerebellar infratentorial and occipital transtentorial.[
Standard management options for CCMs classically include observation and surgical removal.[
Procedures to treat hydrocephalus, such as endoscopic ventriculostomy, should be performed if definitive treatment cannot be carried out immediately.[
Timely microsurgery should be considered several weeks after hemorrhage, to allow perilesional swelling to subside, as well as hematoma contents to evolve and soften, so when there is an emergency situation with hydrocephalus, an ETV should be performed immediately, as well as surgery a few days later.[
The role of radiosurgery in the treatment of cavernous malformations remains controversial. Stereotaxic radiosurgery has been advocated for the treatment of small cavernous angiomas in critical areas. A significant decrease in the bleeding rate has been found after 3 years of treatment.[
CONCLUSION
The ideal treatment is primary lesion removal;[
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Holou WN, O’Lynnger TM, Pandey AS, Gemmete JJ, Thompson BG, Muraszko KM. Natural history and imaging prevalence of cavernous malformations in children and young adults. J Neurosurg Pediatr. 2012. 9: 198-205
2. Burn S, Gunny R, Phipps K, Gaze M, Hayward R. Incidence of cavernoma development in children after radiotherapy for brain tumors. J Neurosurg. 2007. 106: 379-83
3. Cavalcanti DD, Kalani MY, Martirosyan NL, Eales J, Spetzler RF, Preul MC. Cerebral cavernous malformations: From genes to proteins to disease. J Neurosurg. 2012. 116: 122-32
4. Chamadoira C, Cerejo A, Vilarinho A, Castro L, Vaz R. Cavernous malformation of the pineal region. Case report and review of literature Neurocirugía. 2010. 21: 138-45
5. Chang HS, Hongo K, Nakagawa H, Tsuge T. Surgical decision-making on cerebral cavernous malformations. J Clin Neurosci. 2001. 8: 416-20
6. Chenisz JF, Yokoi DS, Fudalli F, Luvison L, Mattozo CA. Surgical treatment of cavernous angiomas in the pineal gland-report of a case. Arq Bras Neurocir. 2018. 37: 242-6
7. Figueiredo A, Maheshwari S, Goel A. Cavernoma in the pineal region. J Clin Neurosci. 2010. 17: 652-3
8. Ghali MG, Srinivasan VM, Mohan AC, Jones JY, Kan PT, Lam S. Pediatric cerebral cavernous malformations: Genetics, pathogenesis, and management. Surg Neurol Int. 2016. 7: S1127-34
9. Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 2011. 30: E24
10. Hankinson EV, Lyons CJ, Hukin J, Cochrane DD. Ophthalmological outcomes of patients treated for pineal region tumors. J Neurosurg Pediatr. 2016. 17: 558-63
11. Kim DS, Shim KW, Kim TG, Chang JH, Park YG, Choi JU. Pineal cavernous malformations: Report of two cases. Yonsei Med J. 2005. 46: 851-8
12. Labauge P, Denier C, Bergametti F, Tournier-Lasserve E. Genetics of cavernous angiomas. Lancet Neurol. 2007. 6: 237-44
13. Lombardi D, Scheithauer BW, Villani RM, Giovanelli M, de Tribolet N. Cavernous haemangioma of the pineal region. Acta Neurochir (Wien). 1996. 138: 678-83
14. Muzumdar DP, Misra BK, Bhaduri AS. Pineal region cavernoma-case report. Neurol Med Chir (Tokyo). 2000. 40: 372-9
15. Ogura T, Kambe A, Sakamoto M, Shinohara Y, Ogawa T, Kurosaki M. Superficial siderosis associated with pineal cavernous malformation. World Neurosurgery. 2018. 109: 230-2
16. Velasquez J, Nieves J, Colasanti R, Collan J, Hernesniemi J. Microsurgical management of vascular malformations of the pineal region. World Neurosurg. 2018. 117: e669-78
17. Vishteh AG, Nadkarni T, Spetzler RF. Cavernous malformation of the pineal region: Short report and review of the literature. Br J Neurosurg. 2000. 14: 147-51