- Department of Neurological Surgery Weill Cornell Medicine, New York City, New York, United States.
- Department of Radiology, Division of Neuroradiology, Weill Cornell Medicine, New York City, New York, United States.
Correspondence Address:
Kashif Majeed, MD, Department of Neurological Surgery, Weill Cornell Medicine, New York City, New York, United States.
DOI:10.25259/SNI_539_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kashif Majeed1, Samuel Z. Hanz1, Michelle Roytman2, J. Levi Chazen2, Jeffrey P. Greenfield1. Identification and surgical ligation of spinal CSF-venous fistula. 11-Oct-2021;12:514
How to cite this URL: Kashif Majeed1, Samuel Z. Hanz1, Michelle Roytman2, J. Levi Chazen2, Jeffrey P. Greenfield1. Identification and surgical ligation of spinal CSF-venous fistula. 11-Oct-2021;12:514. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11169
Abstract
Background: CSF-venous fistulas (CVF) may cause incapacitating positional headaches resulting from spontaneous intracranial hypotension/hypovolemia (SIH). Their etiology remains unknown, although unrecognized local trauma may precipitate SIH. In addition, they are diagnostically challenging despite various imaging tools available. Here, we present CVF identification using magnetic resonance myelography (MRM) and elaborate on their surgical management techniques.
Methods: Retrospective charts of confirmed and treated CVF patients with attention to their diagnostic imaging modalities and management techniques were further reviewed.
Results: Six cases were identified of which three are presented here. There were two females and one male patient. All had fistulas on the left side. Two were at T7-T8 while the third was at T9-T10 level. Two underwent hemilaminotomies at the T7-T8 while the third underwent a foraminotomy at T9 level to access the fistula site. All CVF were closed with a combination of an aneurysm clip and a silk tie. On follow-up, all had complete resolution of symptoms with no evidence of recurrence.
Conclusion: Of the various imaging modalities available, MRM is particularly sensitive in localizing CVF spinal nerve level and their laterality. In addition, the technique of aneurysm clip ligation and placement of a silk tie is curative for these lesions.
Keywords: Clipping, CSF hypovolemia, CSF-venous fistula, Ligation, Magnetic resonance myelography
INTRODUCTION
Spontaneous intracranial hypotension/hypovolemia (SIH) often presents with positional or orthostatic headaches. The positional component may be subtle, evolve gradually, or be absent entirely but is usually part of the diagnosis.[
Schievink et al. classified spontaneous CSF leaks into four categories [
Table 1:
Classification of spontaneous CSF leaks, adopted from Shievink et al.[
SIH due to CVF presents both diagnostic and therapeutic challenges because positional headaches also occur in postural orthostatic tachycardia syndrome,[
Spinal MRI can determine if a large spinal fluid collection is present and may suggest a region of the spine where the leak is located but, in general, cannot pinpoint a leak site. CT myelography (CTM) is the first-line imaging study to diagnose a leak site; however, CTM may be normal in patients with CVF. Hyperdense contrast in the renal collecting systems can suggest an underlying leak but does not help to localize the site.[
MATERIALS AND METHODS
We present three cases of CVF, discuss their management, and review the current CVF literature. Furthermore, we propose a systematic approach to the evaluation and management of this unique and potentially treatable cause of SIH.
RESULTS
Clinical vignettes
Case 1
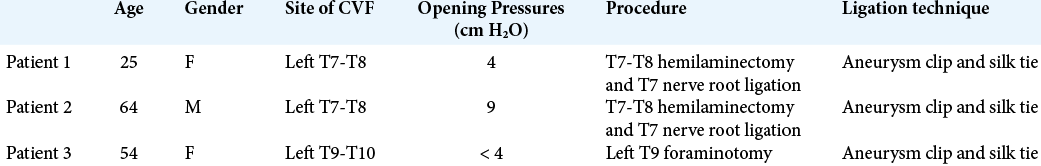
A 25-year-old female presented with a 3-year history of orthostatic headaches with brain MRI findings of pachymeningeal enhancement, brain sagging, pituitary engorgement, and venous distention that were suggestive of SIH. Her CTM was unrevealing. She underwent three blood patches without significant symptomatic improvement. Two years later, she presented with hand numbness, right-sided headaches, and paresthesias of all four extremities and was found to have a large cervicothoracic syrinx. There were no occipital headaches, and a neurological examination was normal except for decreased sensation. She had a Chiari-1 decompressive surgery that did not relieve her symptoms. She underwent a magnetic resonance myelography (MRM) that revealed a T7-T8 meningeal diverticulum and an enhancing left paraspinal vein draining into the azygos system [
Surgical procedure and follow-up
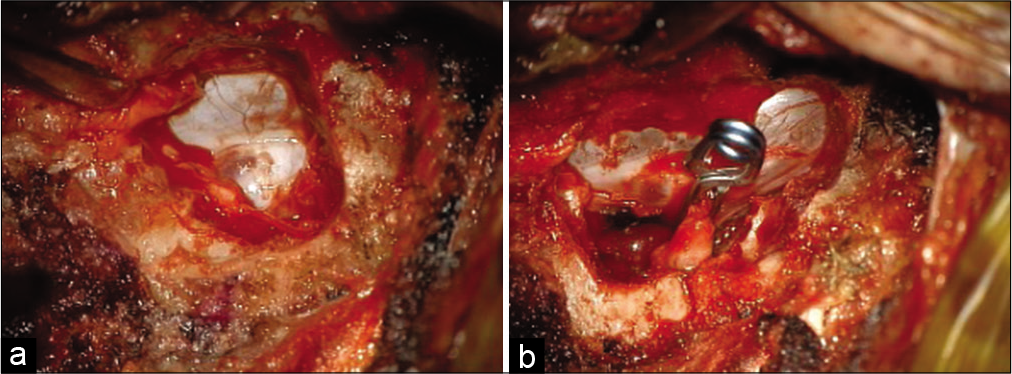
She was brought to the operating room supine, intubated with arterial and venous access established. After preoperative antibiotics and steroids administration, fluoroscopic guidance was utilized to localize the T7-T8 interspace where the T7 nerve root exits. The access was gained through a prior surgical incision, which was extended superiorly and just lateral to the dural sac to expose the nerve root complex. There was significant venous engorgement, which was coagulated and dissected around the nerve root to make room for placing a temporary aneurysm. During the 10 min wait, the somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEP) had remained stable. A permanent aneurysm clip was then placed and further reinforced with a zero-silk tie around the nerve root. The area was thoroughly irrigated with antibiotic solution and packed with muscle and Tisseel. The wound was closed in layers using 3-0 Vicryl for deeper layers and with subcuticular 4-0 Caprosyn. She tolerated the procedure well; her intraoperative electrophysiological monitoring remained stable throughout the procedure. She was extubated and transferred to the recovery room.
The patient’s headaches, hand numbness gradually resolved, and within 3 months, she was asymptomatic. Repeat MRI showed a complete resolution of the syringomyelia and ascent of the herniated cerebellar tissue [
Case 2
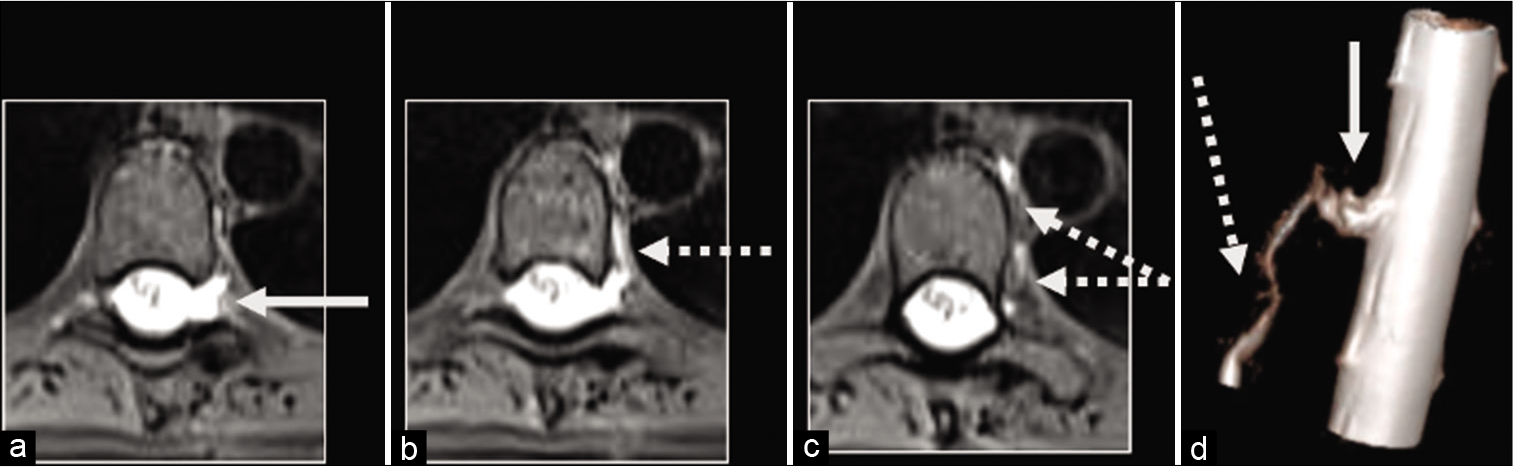
A 64-year-old male presented with a prolonged history of migraines and severe vertex headaches aggravated by bending, coughing, sneezing, and straining. His positional symptoms and MRI brain findings (SEEPS) suggested SIH. He received a short course of steroids and two blood patches without relief of his symptoms. On MRM, he was found to have a left T7-T8 spinal meningeal diverticulum, a vascular malformation of the lower thoracic spine, and contrast opacification extending along the left paraspinal vein into the azygous system, representing a CVF [
Surgical procedure and follow-up
As described above, after intubation, arterial and venous access, and administration of preoperative antibiotics, the fluoroscopic guidance was utilized to identify the small coil placed during the preoperative angiogram. The level was further confirmed by counting vertebral bodies and ribs corresponding to the level of the coil. Through a midline incision, the dissection was performed along the spinous process over the lamina, above and below the targeted nerve root of the fistula. Using a Cloward retractor, lamina was exposed, and the hemilaminectomy from outside to inside fashion was performed to identify the medial dura of the thecal sac. Kerrison punches were used to unroof the bone up to expose the lesion with large veins and the dilated nerve. Under the operating microscope, enough space was created around the lesion allowing for two 10 mm straight aneurysm clips to be placed to disrupt the connection between the nerve root and the vessel. After a wait of 10 min to confirm that MEPs and SSEPs remained normal, a permanent aneurysm clip was placed, which was further reinforced with a 2-0 silk tie.
The dilated nerve root was further cauterized, and the area was packed with muscle, and the dural sealant was applied. After copious antibiotic irrigation, the wound was closed in layers using 3-0 Vicryl sutures and subcuticular 4-0 Caprosyn for the skin. He was extubated and transferred to the recovery room. On follow-up, his severe headaches and tinnitus had resolved [
Case 3
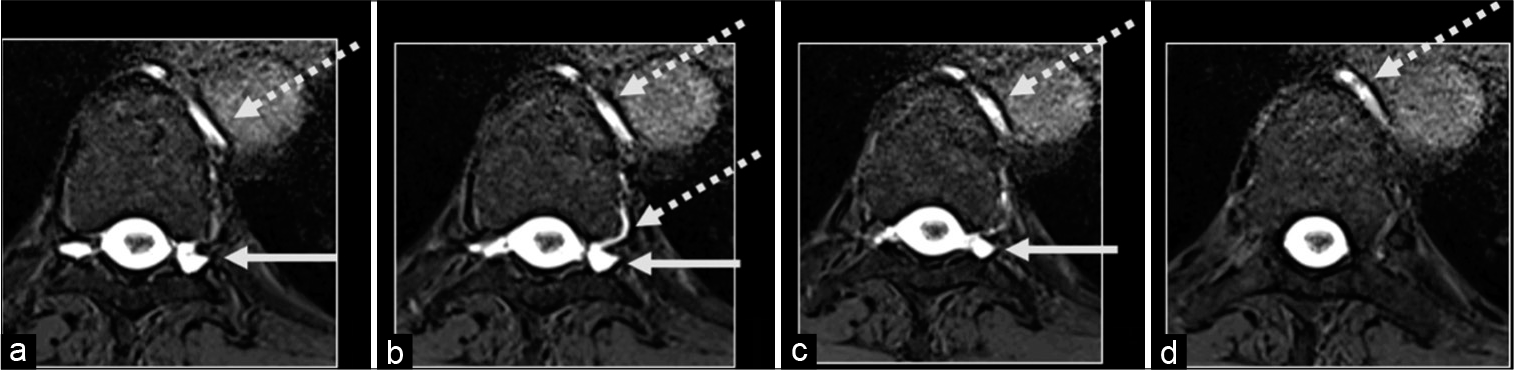
A 54-year-old female presented with unremitting headaches, and brain MRI findings (SEEPS) were suggestive of SIH. She underwent multiple blood patches without relief of her symptoms. An MRM was performed, revealing bilateral extensive spinal meningeal diverticula with a possible CVF at T9-10, for which she underwent a right T9 nerve root ligation. However, her symptoms recurred shortly thereafter, and a follow-up CTM demonstrated a left T9-T10 spinal meningeal diverticulum with opacification of an adjacent left paraspinal vein, suspicious for a CVF [
Surgical procedure and follow-up
Since the patient had prior surgery on the right side at that level, the same incision was used to gain access down to the fascia. Fluoroscopic guidance was used to identify the level utilizing the clip as a marker that was placed on the contralateral side. The level was further confirmed using the visual anatomic landmarks, identifying the left TP at T9, and dissected laterally without identifying the dura in the midline to a straight lateral approach. The entire surgery was performed under the operative microscope, the T9-T10 joint space corresponding to the nerve root was identified, and a small bony window was created. Hemostasis using bone wax and coagulation of epidural veins was achieved, and the plane around the nerve root was cleared.
A large straight nonfenestrated clip across the nerve root complex was applied. The MEPs and SSEPs remained stable during the 5 min wait time; therefore, the clip was left in place. The venous structures lateral and deep to the nerve root clip were further coagulated, and the area was packed with muscle and Tisseel to obliterate the region completely. The area was copiously irrigated with antibiotic solution and then closed in layers, using Vicryl 0 and 2-0 for deep layers and the skin with 3-0 Vicryl and a 4-0 Caprosyn. She tolerated the procedure well, was rolled onto her back, extubated, and moved to the recovery room without any complications.
Her postoperative recovery has been unremarkable, and all her symptoms have completely resolved. Her tonsillar herniation persists, but she remains asymptomatic [
DISCUSSION
Spontaneous CVF was first described in a small case series as a cause of SIH.[
According to recent estimates, spinal CVF may represent between 2.5% and 20% of SIH cases.[
In addition, a series of 22 cases also found that 90% of them lacked clear CSF leak, and 82% were associated with a dural sleeve diverticulum.[
CVF may be associated with a single draining vein or a network of dilated veins surrounding the spinal nerve root sleeve in the form of a diverticulum leading to the rapid absorption of extravasated CSF within the venous system.[
Since CVF are a potentially treatable cause of protracted SIH, we propose actively looking for an enhancing paraspinal vein when performing CTM or MRM, which is reported to be an important diagnostic sign.[
In a series of 53 patients, Schievink et al. made a case for lateral decubitus DSM due to its rapid imaging and high temporal and spatial resolution when compared to conventional imaging. In addition, they found that conventional CTM or MRI failed to locate the CSF leak in the majority (80%) of cases. Ten of the 53 patients in their series were confirmed to have CVF, concluding the prevalence to be about 19%, which may be an overestimation due to selection bias. Similar to the findings of Kranz et al.,[
The literature describes the two techniques used to delineate leaks are “initial” and “problem-solving.” Various authors note CTM to be the initial technique that helps approximate the lesion, whereas problem-solving techniques such as DM and DSM help pinpoint the lesion.[
It has also been reported in a small case series that CVF present with accompanying venous or venolyphatic vascular malformations.[
There is little consensus in the literature on how to best diagnose these CVF; some authors suggest lateral decubitus CTM, whereas others propose DSM to increase their detection. However, both of these techniques expose the patient to a substantial amount of ionizing radiation, and DSM is operator-dependent. In contrast, we were able to localize CVF using MRM by off-label but well-tolerated gadolinium injection intrathecally, under CT guidance, thereby preventing any extensive deleterious effects resulting from this approach. The patient was rolled multiple times after the starting Trendelenberg position to disperse the contrast in the subarachnoid space before transferring to the MR suite to perform MRM on a 3T Biograph mMR scanner (Siemens). Multiple fat-suppressed T1 sequences were reviewed in real-time as well as delayed thin-section axial sequences were obtained.[
On the other hand, if no enhancing paraspinal vein or a leak is identified on MRM, other diagnoses should be considered. If a leak is identified, the patient should receive a trial of a blood patch or fibrin glue. If, however, a leak is not identified, follow-up DSM should take place. Alternatively, if no HDPSV is found, but a leak is identified, proceed directly to blood patch or fibrin glue injection because the likelihood of a CVF is low and the chance of sealing the dural defect causing SIH is not insignificant. However, if the patient continues to have symptoms, definitive treatment endovascularly or with surgical repair, as shown below, should follow [
The use of aneurysm clips has emerged as an excellent treatment choice that prevents needle holes encountered while stitching the dura. Prior authors have suggested “ligating”[
Fistula clipping in our series was performed by first locating the fistula site using MRM. A preoperative angiogram identified the location of the artery of Adamkiewicz, and a coil was placed to mark the fistula site. Dissection was performed as medially as possible lateral to the dural thecal sac. Given that venous engorgement may occlude visualization of the fistula, these veins were coagulated, dissected with a Rhoton instrument, and the nerve hooks were placed around the nerve root complex in this instance. Case 1 exemplifies this. Once located, the fistula was clipped using a temporary aneurysm clip followed by neuromotor monitoring to confirm intact function [
On follow-up, patients reported complete resolution of their preoperative symptoms, suggesting that clipping the fistula with a permanent aneurysm clip with or without a 2-0 silk suture reinforcement shows promise in treating SIH caused by CVF. Although our cohort is small, we argue that ligation of the fistula with preferably two forms of occlusion, with clipping being the predominant form, shows promise in permanent amelioration of symptoms.
CONCLUSION
We present three patients treated for SIH caused by CVF and discuss their clinical presentation, diagnostic challenges, and management in the light of current literature. A systematic approach leads to a search for enhancing paraspinal vein and direct visualization using real-time MRM. This approach can obviate the need for unnecessary surgery. We found MRM to be quite helpful in visualizing the HDPSV as well as locating the fistula site. We propose using DM/DSM in only those cases where MRM has been inconclusive in resolving the site of the fistula. The intraoperative localization can be further aided by placing a coil at the fistula site during preoperative angiography. The definitive surgical management should proceed with ligation of the defect using one or more permanent aneurysm clips after ensuring normal intraoperative monitory when temporary aneurysm clips are placed. Reinforcement with a 2-0 silk suture adds another layer of securing the fistula to permanently ameliorate patients’ symptoms. This technique seems to have long-term efficacy in ameliorating symptoms of SIH.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chazen JL, Robbins MS, Strauss SB, Schweitzer AD, Greenfield JP. MR myelography for the detection of CSFvenous fistulas. AJNR Am J Neuroradiol. 2020. 41: 938-40
2. Cho KI, Moon HS, Jeon HJ, Park K, Kong DS. Spontaneous intracranial hypotension: Efficacy of radiologic targeting vs blind blood patch. Neurology. 2011. 76: 1139-44
3. Clark MS, Diehn FE, Verdoorn JT, Lehman VT, Liebo GB, Morris JM. Prevalence of hyperdense paraspinal vein sign in patients with spontaneous intracranial hypotension without dural CSF leak on standard CT myelography. Diagn Interv Radiol. 2018. 24: 54-9
4. Dobrocky T, Grunder L, Breiding PS, Branca M, Limacher A, Mosimann PJ. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol. 2019. 76: 580-7
5. Duvall JR, Robertson CE, Cutsforth-Gregory JK, Carr CM, Atkinson JL, Garza I. Headache due to spontaneous spinal cerebrospinal fluid leak secondary to cerebrospinal fluid-venous fistula: Case series. Cephalalgia. 2019. 39: 1847-54
6. Farb RI, Nicholson PJ, Peng PW, Massicotte EM, Lay C, Krings T. Spontaneous intracranial hypotension: A systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol. 2019. 40: 745-53
7. Kranz PG, Amrhein TJ, Gray L. CSF venous fistulas in spontaneous intracranial hypotension: Imaging characteristics on dynamic and CT myelography. AJR Am J Roentgenol. 2017. 209: 1360-6
8. Kranz PG, Amrhein TJ, Schievink WI, Karikari IO, Gray L The. “hyperdense paraspinal vein” sign: A marker of CSF-venous fistula. AJNR Am J Neuroradiol. 2016. 37: 1379-81
9. Kranz PG, Gray L, Malinzak MD, Amrhein TJ. Spontaneous intracranial hypotension: Pathogenesis, diagnosis, and treatment. Neuroimaging Clin N Am. 2019. 29: 581-94
10. Kranz PG, Luetmer PH, Diehn FE, Amrhein TJ, Tanpitukpongse TP, Gray L. Myelographic techniques for the detection of spinal CSF leaks in spontaneous intracranial hypotension. AJR Am J Roentgenol. 2016. 206: 8-19
11. Kranz PG, Malinzak MD, Amrhein TJ, Gray L. Update on the diagnosis and treatment of spontaneous intracranial hypotension. Curr Pain Headache Rep. 2017. 21: 37
12. Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension?. Cephalalgia. 2016. 36: 1209-17
13. Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L. Imaging signs in spontaneous intracranial hypotension: Prevalence and relationship to CSF pressure. AJNR Am J Neuroradiol. 2016. 37: 1374-8
14. Kumar N, Diehn FE, Carr CM, Verdoorn JT, Garza I, Luetmer PH. Spinal CSF venous fistula: A treatable etiology for CSF leaks in craniospinal hypovolemia. Neurology. 2016. 86: 2310-2
15. Kumar N, Neidert NB, Diehn FE, Campeau NG, Morris JM, Bjarnason H. A novel etiology for craniospinal hypovolemia: A case of inferior vena cava obstruction. J Neurosurg Spine. 2018. 29: 452-5
16. Kumar Y, Hooda K, Li S, Karol I, Muro GJ. A case of spontaneous intracranial hypotension: The role of dynamic CT myelography and epidural blood patch in diagnosis and treatment. Conn Med. 2015. 79: 547-9
17. Luetmer PH, Schwartz KM, Eckel LJ, Hunt CH, Carter RE, Diehn FE. When should I do dynamic CT myelography? Predicting fast spinal CSF leaks in patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2012. 33: 690-4
18. Maillot C. The space surrounding the spinal cord. Constitution organization and relationship with the cerebrospinal fluid. J Radiol. 1990. 71: 539-47
19. Mokri B, Low PA. Orthostatic headaches without CSF leak in postural tachycardia syndrome. Neurology. 2003. 61: 980-2
20. Mokri B. Headaches caused by decreased intracranial pressure: Diagnosis and management. Curr Opin Neurol. 2003. 16: 319-26
21. Mokri B. Spontaneous CSF leaks: Low CSF volume syndromes. Neurol Clin. 2014. 32: 397-422
22. Mokri B. Spontaneous low cerebrospinal pressure/volume headaches. Curr Neurol Neurosci Rep. 2004. 4: 117-24
23. Schievink WI, Maya MM, Jean-Pierre S, Nuno M, Prasad RS, Moser FG. A classification system of spontaneous spinal CSF leaks. Neurology. 2016. 87: 673-9
24. Schievink WI, Maya MM, Moser FG, Prasad RS, Cruz RB, Nuno M. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine. 2019. p. 1-4
25. Schievink WI, Maya MM, Moser FG, Tuchman A, Cruz RB, Farb RI. Spontaneous spinal CSF-venous fistulas associated with venous/venolymphatic vascular malformations: Report of 3 cases. J Neurosurg Spine. 2019. 32: 305-10
26. Schievink WI, Maya MM, Moser FG. Digital subtraction myelography in the investigation of post-dural puncture headache in 27 patients: Technical note. J Neurosurg Spine. 2017. 26: 760-4
27. Schievink WI, Maya MM, Moser FG. False localizing signs of spinal CSF-venous fistulas in spontaneous intracranial hypotension: Report of 2 cases. J Neurosurg Spine. 2019. p. 1-4
28. Schievink WI, Morreale VM, Atkinson JL, Meyer FB, Piepgras DG, Ebersold MJ. Surgical treatment of spontaneous spinal cerebrospinal fluid leaks. J Neurosurg. 1998. 88: 243-6
29. Schievink WI, Moser FG, Maya MM, Prasad RS. Digital subtraction myelography for the identification of spontaneous spinal CSF-venous fistulas. J Neurosurg Spine. 2016. 24: 960-4
30. Schievink WI, Moser FG, Maya MM. CSF-venous fistula in spontaneous intracranial hypotension. Neurology. 2014. 83: 472-3
31. Schievink WI, Schwartz MS, Maya MM, Moser FG, Rozen TD. Lack of causal association between spontaneous intracranial hypotension and cranial cerebrospinal fluid leaks. J Neurosurg. 2012. 116: 749-54
32. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006. 295: 2286-96
33. Schonberger J, Mohlenbruch M, Seitz A, Bussmann C, Bachli H, Kolker S. Chiari-like displacement due to spontaneous intracranial hypotension in an adolescent: Successful treatment by epidural blood patch. Eur J Paediatr Neurol. 2017. 21: 678-81
34. Sencakova D, Mokri B, McClelland RL. The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology. 2001. 57: 1921-3
35. Shah LM, McLean LA, Heilbrun ME, Salzman KL. Intracranial hypotension: Improved MRI detection with diagnostic intracranial angles. AJR Am J Roentgenol. 2013. 200: 400-7
36. Wang TY, Karikari IO, Amrhein TJ, Gray L, Kranz PG. Clinical outcomes following surgical ligation of cerebrospinal fluid-venous fistula in patients with spontaneous intracranial hypotension: A prospective case series. Oper Neurosurg (Hagerstown). 2020. 18: 239-45