- Department of Neurosurgery, University of Tsukuba, Tsukuba, Japan.
- Department of Neurosurgery, Tsukuba Memorial Hospital, Tsukuba, Japan.
Correspondence Address:
Eiichi Ishikawa, Department of Neurosurgery, University of Tsukuba, Tsukuba, Japan.
DOI:10.25259/SNI_789_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Atsushi Tsukada1, Kiyoyuki Yanaka2, Hayato Takeda2, Kuniyuki Onuma2, Maya Takada2, Kazuhiro Nakamura2, Eiichi Ishikawa1. Idiopathic focal calvarial thinning: A case report. 04-Nov-2022;13:503
How to cite this URL: Atsushi Tsukada1, Kiyoyuki Yanaka2, Hayato Takeda2, Kuniyuki Onuma2, Maya Takada2, Kazuhiro Nakamura2, Eiichi Ishikawa1. Idiopathic focal calvarial thinning: A case report. 04-Nov-2022;13:503. Available from: https://surgicalneurologyint.com/surgicalint-articles/11982/
Abstract
Background: Calvarial bone thinning is a rare clinical entity, with only several cases reported (including Gorham-Stout disease), but the cause is often unknown. Here, we report such a case of unilateral calvarial thinning with an unknown cause.
Case Description: A 77-year-old woman undergoing imaging examination for unruptured cerebral aneurysms for the past several years noticed a progressive cranial deformity. Computed tomography revealed progressive thinning of the right parietal bone and cranial deformity but laboratory tests showed no causative findings. A cranioplasty was performed to protect the brain and confirm the pathology. Grossly, pigmentation and deformity were observed on the outer plate of the bone but the inner plate was intact. Pathological examination revealed preserved bone cells and no necrosis. In addition, there were no findings of vascular hyperplasia or malignancy. It appeared that localized osteoporosis had occurred, mainly in the outer plate of the bone, but the cause was unclear.
Conclusion: Progressive focal calvarial thinning is rarely reported and the mechanism in this case was unknown. It is important to determine the cause of the bone thinning to evaluate the need for surgical intervention from the viewpoint of brain protection and prevention of cerebrospinal fluid leakage.
Keywords: Calvarial thinning, Idiopathic, Parietal bone
INTRODUCTION
Bone mass is tightly balanced between osteoblast-mediated formation and bone resorption by osteoclasts. When this balance is disrupted, bone thickening and thinning occur.[
CASE DESCRIPTION
A 77-year-old woman was referred to our hospital because of a recent and noticeable skull deformity. The patient also had a small and unruptured middle cerebral artery aneurysm regularly examined by magnetic resonance imaging but no other intracranial lesions had since emerged. The patient had a history of well-controlled hypertension but no other significant medical history, including diabetes or head trauma. No relatives had skull deformities.
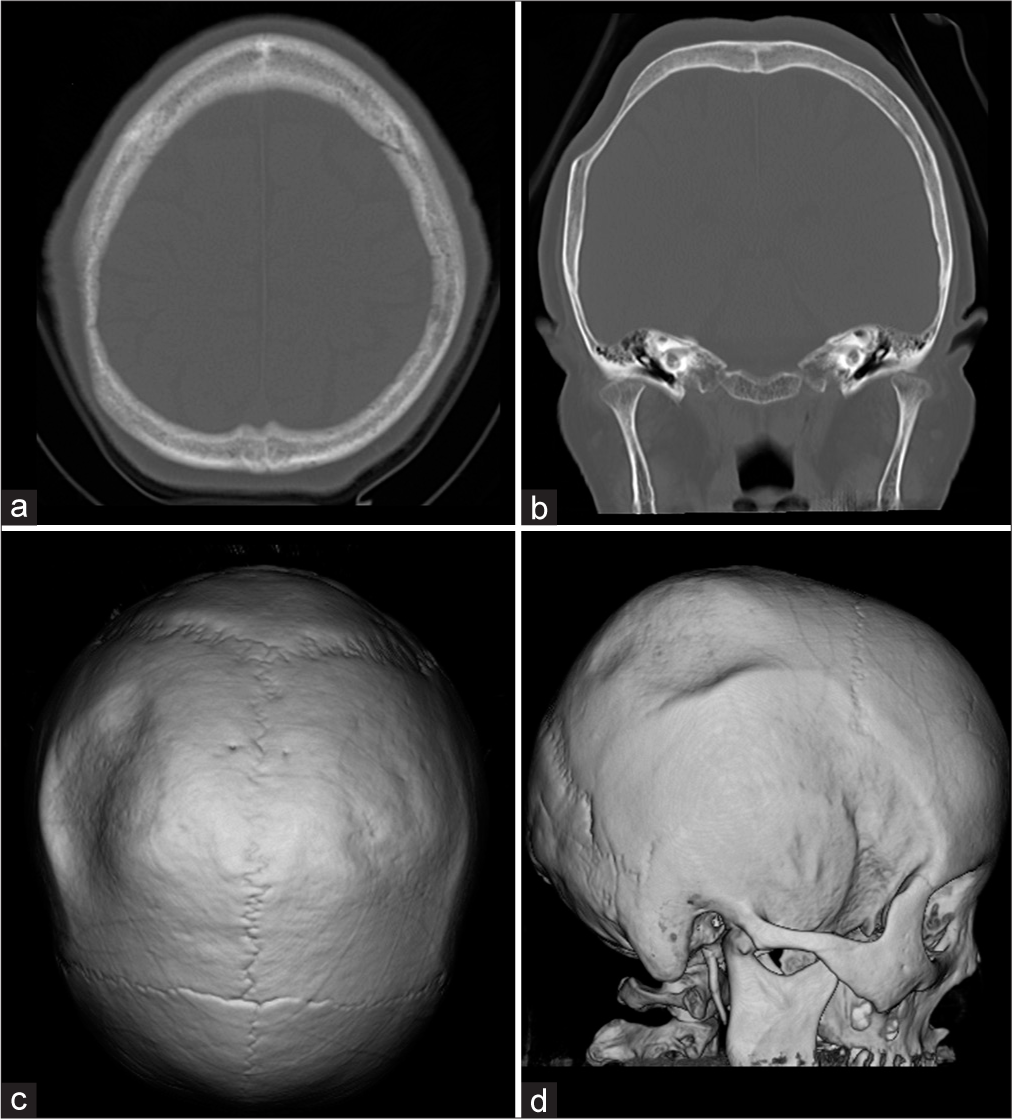
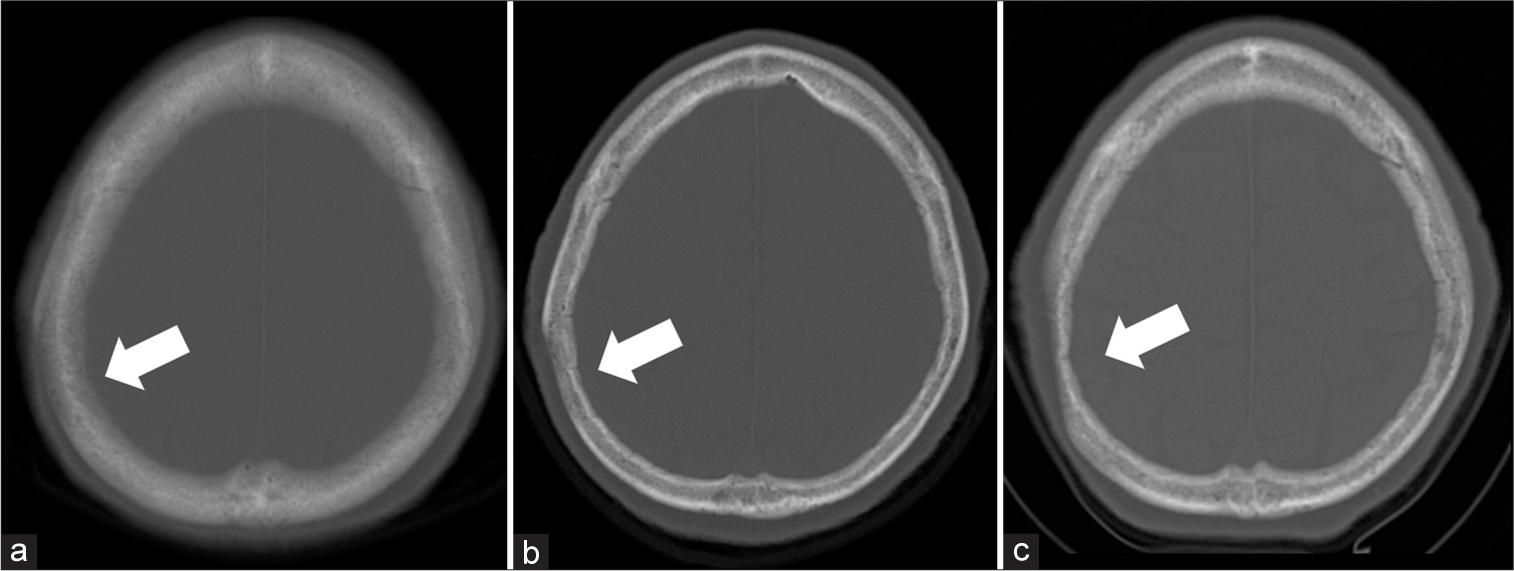
Physical examination on admission revealed a 6 × 10 cm depressed deformity on the right parietal region but the skin directly above was normal with no tenderness in the deformed area. Computed tomography (CT) showed that the skull of the right parietal bone was thin but no significant neoplastic lesions are shown in [
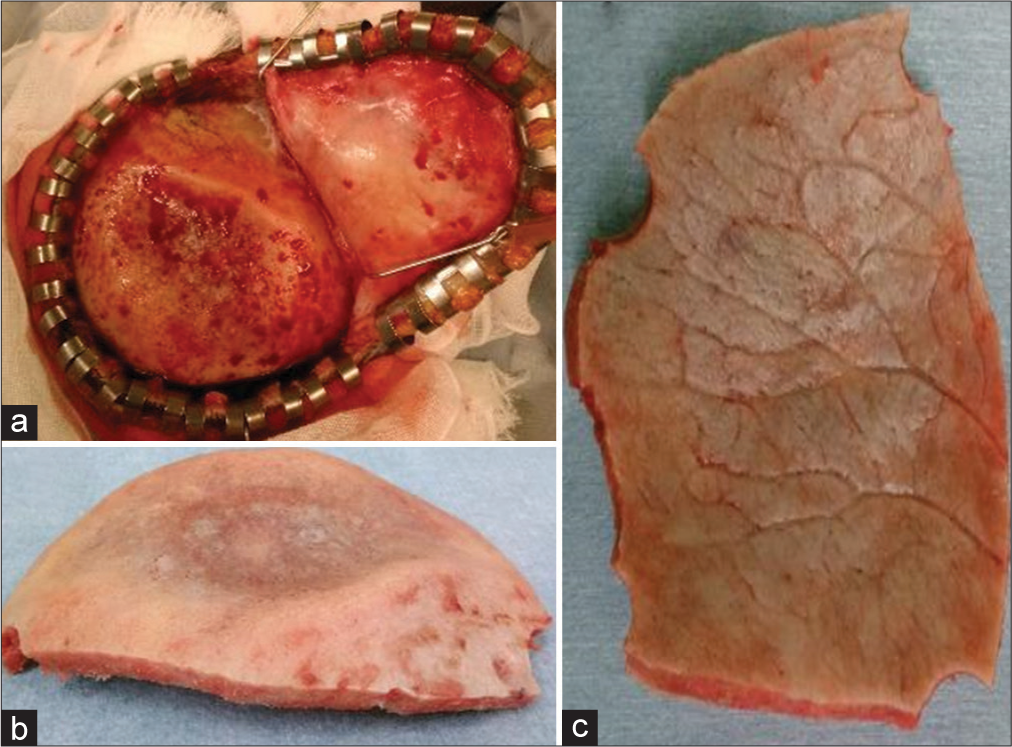
The patient was cosmetically concerned about the skull deformity. Furthermore, from the viewpoint of the need for pathological examination and since there was not enough bone to protect the brain, a cranioplasty was performed after excising the thinning bone. When the skin over the depressed area was turned over, there was an area of 6 × 10 cm in the right parietal bone that was thinned out and was creating a small crater in the calvaria [
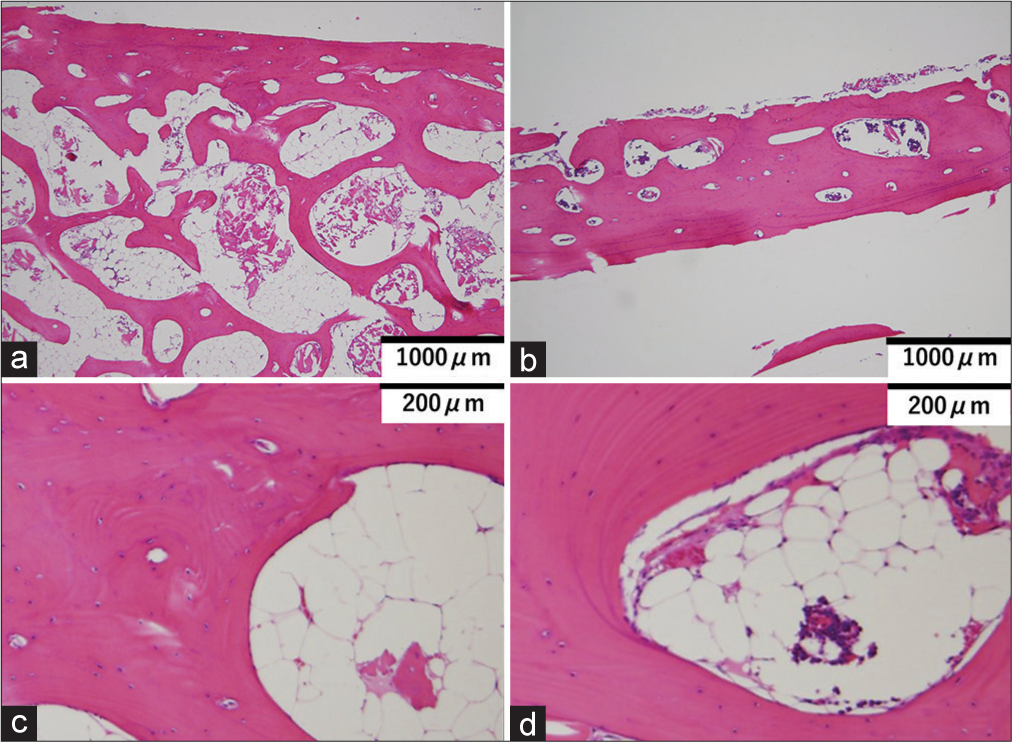
Pathological examination showed a significant reduction in cancellous bone in the thinned area. Cortical bone volume was slightly decreased in the outer plate but was almost normal in the inner plate. No lymphatic vessel proliferation, malformation, or lymphocyte proliferation was observed, nor was there any obvious tumor, inflammation, osteolysis, or necrosis [
Figure 4:
Pathological specimens of the normal part (a and c) and thinning part (b and d) are shown. In the low magnification area (b), there is a decrease in bone volume, mainly in the cavernous bone, in the thinned areas. However, no obvious cellular abnormalities exist even at high magnification (d). No lymphatic vessel proliferation, malformation, or lymphocyte proliferation is observed, nor is there any obvious tumor, inflammation, osteolysis, or necrosis.
DISCUSSION
Calvarial thinning can occur during various pathologies both as a primary manifestation in the skull and as a secondary involvement of trauma and various systemic diseases, such as primary and metastatic tumors inducing intracranial hypertension, hyperparathyroidism, inflammatory disease, diabetes mellitus, osteomyelitis, systemic mastocytosis, prolonged steroid therapy, bone aneurysm, or cystic angiomatosis of bone.[
In this case, with almost normal laboratory tests and no obvious genetic history, it was necessary to speculate on other causes, such as GSD and Winchester syndrome. GSD, also called “vanishing/phantom bone disease,” is characterized by spontaneous and progressive osteolytic lesions in a single bone or multiple bones. The most commonly involved sites are the mandible, followed by the scapula, ribs, humerus, pelvis, and femur while the bones of the skull are rarely affected.[
Osteolysis is most likely due to the nonmalignant growth of hemangiomatous tissue.[
While, in cases of idiopathic bone loss, it is challenging to select the most appropriate treatment, surgery may be essential to prevent secondary disorders such as cerebrospinal fluid leakage[
CONCLUSION
We report a case of idiopathic focal calvarial thinning with an unclear cause. Progressive calvarial thinning is rarely reported and the mechanism in this case was unknown. However, it is important to determine the cause of the bone thinning to evaluate the need for surgical intervention from the viewpoint of brain protection and prevention of cerebrospinal fluid leakage.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. de Keyser CE, Saltzherr MS, Bos EM, Zillikens MC. A large skull defect due to Gorham-stout disease: Case report and literature review on pathogenesis, diagnosis, and treatment. Front Endocrinol (Lausanne). 2020. 11: 37
2. Evans BR, Mosig RA, Lobl M, Martignetti CR, Camacho C, Grum-Tokars V. Mutation of membrane Type-1 metalloproteinase, MT1-MMP, causes the multicentric osteolysis and arthritis disease Winchester syndrome. Am J Hum Genet. 2012. 91: 572-6
3. Lo PC, Chen CY, Chin SC, Juan CJ, Hsueh CJ, Chen A. Disappearing calvarium in Gorham disease: MR imaging characteristics with pathologic correlation. AJNR Am J Neuroradiol. 2004. 25: 415-8
4. Lyer GV. Cerebrospinal fluid rhinorrhoea from massive osteolysis of the skull. J Neurol Neurosurg Psychiatry. 1979. 42: 767-9
5. Rabbani C, Saltagi MZ, Ye MJ, Patel JM, Manchanda S, Nelson RF. Association of obstructive sleep apnea with calvarial and skull base thinning. JAMA Otolaryngol Head Neck Surg. 2018. 144: 513-8
6. Takaya K, Sakamoto Y, Miwa T, Yoshida K, Kishi K. Gorham-Stout disease with parietal bone osteolysis: A case series and review of literature. Br J Neurosurg. 2021. 35: 27-31
7. Takeda S. Regulatory mechanism of bone metabolism. Nichijinkaisi (Japanese). 2014. 56: 1188-95
8. Tsutsumi A, Yasumoto Y, Ito M. Idiopathic calvarial thinning. Neurol Med Chir (Tokyo). 2008. 48: 275-8