- Department of Spinal Neurosurgery, Kyoto-Katsura Hospital, Kyoto, Japan.
- Department of Neurosurgery, Hikone Chuo Hospital, Hikone, Japan
- Department of Neurosurgery, Kurashiki Central Hospital, Kurashiki, Japan
- Department of Neurosurgery, Kobe City Medical Center General Hospital, Kobe City, Japan
- Department of Neurosurgery, Japanease Red Cross Otsu Hospital, Otsu, Japan.
Correspondence Address:
Tamaki Kobayashi, Department of Spinal Neurosurgery, Kyoto-Katsura Hospital, Kyoto, Japan.
DOI:10.25259/SNI_157_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tamaki Kobayashi1, Yosinori Maki2, Hiroyuki Ikeda3, Masaomi Koyanagi4, Masashi Oda5, Masaaki Saiki5. Immunoglobulin G4-related disease manifesting as peripheral neuropathy: A rare clinical symptom due to rare autoimmune disease. 14-Jun-2024;15:197
How to cite this URL: Tamaki Kobayashi1, Yosinori Maki2, Hiroyuki Ikeda3, Masaomi Koyanagi4, Masashi Oda5, Masaaki Saiki5. Immunoglobulin G4-related disease manifesting as peripheral neuropathy: A rare clinical symptom due to rare autoimmune disease. 14-Jun-2024;15:197. Available from: https://surgicalneurologyint.com/surgicalint-articles/12944/
Abstract
Background: Nervous system involvement in immunoglobulin G4-related disease (IgG4-RD) has been rarely reported.
Case Description: We describe an unusual case of IgG4-RD manifested as paresthesia in the right lower extremity. A 51-year-old male presented with paresthesia in the right S1–S3 regions. A neurological examination revealed peripheral neuropathy. Blood examination results were normal, barring slightly elevated IgG levels. Initial magnetic resonance imaging of the swollen right S1 and S2 nerve roots revealed lymphoma, schwannoma, and sarcoidosis. However, following the biopsy, the pathological findings were not typical of these diseases. Abdominal computed tomography revealed perirenal lesions, and IgG4-RD was suspected. The patient had a serum IgG4 level of 724 mg/dL. Additional pathological evaluations of the swollen S1 nerve revealed findings that corresponded to the diagnostic criteria for IgG4-RD. Oral steroid therapy was initiated, which improved paresthesia, and the swollen S1 nerve root gradually shrank.
Conclusion: This report highlights a rare case of IgG4-RD involving nerve roots that neurosurgeons should consider.
Keywords: Immunoglobulin G4-related disease, Nerve root, Pathology, Peripheral neuropathy, Spine
INTRODUCTION
Immunoglobulin G4-related disease (IgG4-RD) is characterized by tumefactive lesions from underlying autoimmune fibroinflammatory conditions, mainly involving the pancreas, bile duct, lungs, and rarely the nervous system.[
CASE PRESENTATION
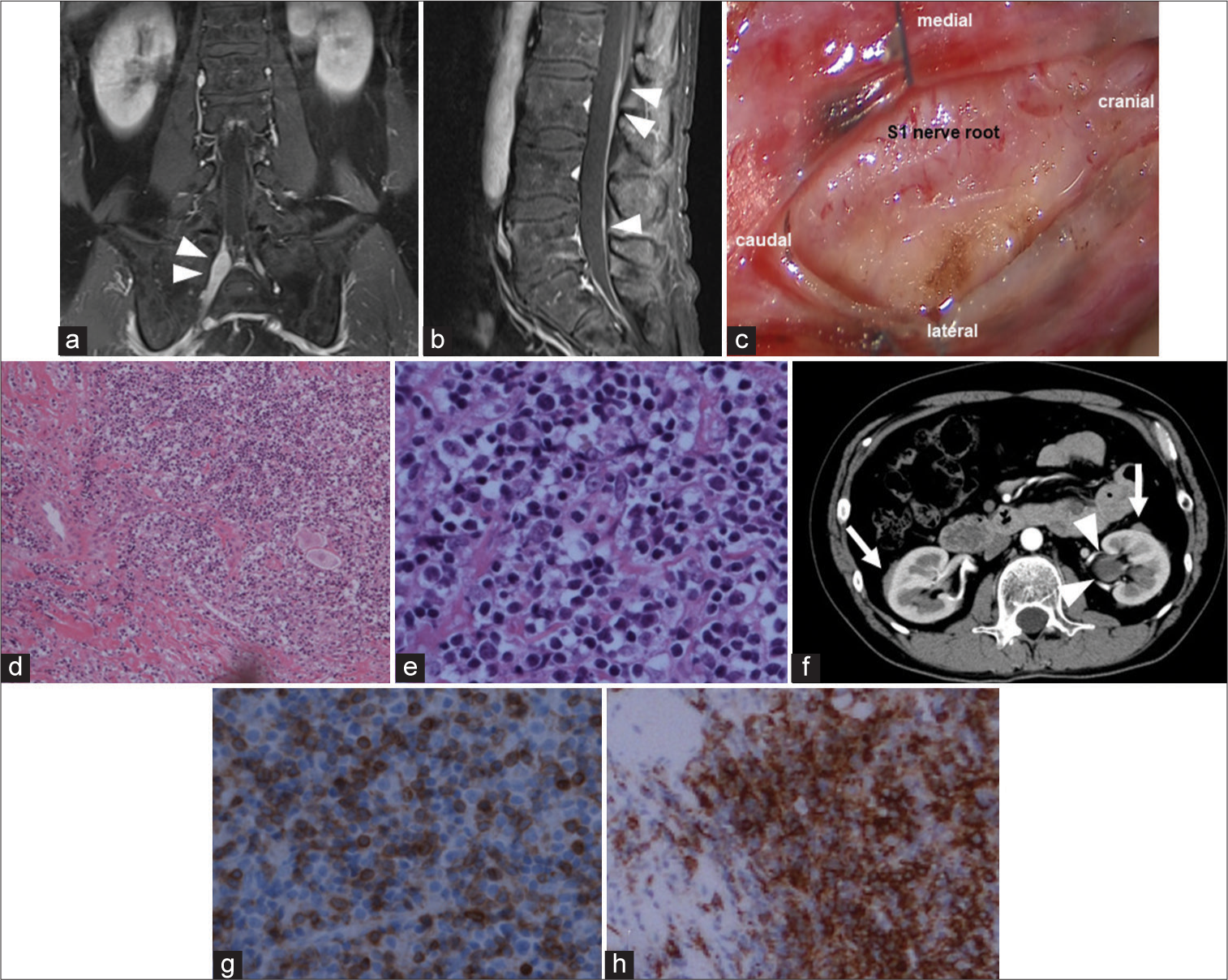
A 51-year-old male presented to our neurosurgery department complaining of numbness in the right lower extremity that had gradually worsened over a year. He had undergone surgery for pneumothorax and had varicose veins and emphysema but was not taking any medication. Neurological examination revealed paresthesia in the medial-posterior side of the right leg and buttock and the absence of the right Achilles tendon reflex. The paresthesia region appeared to correspond to the dermatome of left S1– S3. No neurological deficits were observed. We hypothesized that the patient’s neurological symptoms could be attributed to peripheral neuropathy. Blood and cerebrospinal fluid samples revealed no abnormalities in rheumatoid factor, antinuclear antibodies, human immunodeficiency virus, syphilis antibodies, or malignancies. Serum IgG levels were slightly elevated at 1974 mg/dL (normal range: 870–1700 mg/dL). Pelvic magnetic resonance imaging (MRI) was performed to rule out paresthesia caused by the mass. The right S1 and S2 nerve roots appear swollen and homogeneously enhanced on T1-weighted gadolinium-enhanced imaging. The dura appeared partially hypertrophic and enhanced [
Figure 1:
(a and b) The right S1 and S2 nerve roots (white arrowheads) appear swollen and enhanced with the contrast agent. The hypertrophic lumbar dura (white arrowhead) appears partially enhanced (a) coronal T1-weighted gadolinium-enhanced image and (b) sagittal T1-weighted gadolinium-enhanced image. (c) Intraoperatively, reddish and swollen S1 nerve roots can be observed on opening the dura; (d and e) lymphocyte aggregation was accompanied by the diffuse growth of fibrous tissue (hematoxylin-eosin staining with original magnification ×100 (d) and original magnification ×400 (e). (f and g) The specimen is positive for CD3 (original magnification, ×400) and CD20 (original magnification, ×200). (h)The left dilated ureter (white arrowheads) and bilateral perirenal lesions (white arrows) can be observed on contrast-enhanced computed tomography.
Figure 2:
(a and b) The specimen of the right S1 nerve exhibits dense lymphoplasmacytic infiltration, obliterative phlebitis, and storiform growth of collagen fibers (a and b: hematoxylin-eosin staining, original magnification ×100). (c and d) The specimen is positive for immunoglobulin G (IgG) (c: original magnification ×200) and IgG4 (d: original magnification ×200), and more than half of the tissue positive for IgG is positive for IgG4. (e-g) The ureter specimen shows similar findings on hematoxylin-eosin staining (e), immunostaining of IgG (f), and that of IgG4 (g) (original magnification ×200).
DISCUSSION
Here, we describe a rare case of IgG4-RD involving the S1 and S2 nerve roots. After initially suspecting peripheral neuropathy due to paresthesia in the right S1-S3 region, biopsy and pathological examination of the involved S1 nerve root and perirenal lesion revealed IgG4-RD. Due to the rarity of IgG4-RD involving the nerve roots, diagnosis can be challenging.
Nervous system involvement is a rare manifestation of IgG4-RD.[
The diagnostic criteria for IgG4-RD include one or more organs manifesting a nodular or swollen shape (lymph node swelling should be omitted), elevated serum IgG4 level (>135 mg/dL), and pathological diagnosis.[
CONCLUSION
Neurosurgeons should consider the possibility of a rare entity, such as IgG4-RD, that mimics peripheral neuropathy accompanied by atypical radiological findings. To rule out IgG4-RD occurring in rare regions such as nerve roots, abdominal CT screening can aid in the diagnosis of typical IgG4-RD-related findings.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
This article is dedicated to the late Dr. Yoko Okamoto. We appreciate her valuable collaboration in the pathological diagnosis of this report.
References
1. Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012. 25: 1181-92
2. Inoue D, Zen Y, Sato Y, Abo H, Demachi H, Uchiyama A. IgG4-related perineural disease. Int J Rheumatol. 2012. 2012: 401890
3. Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015. 67: 1688-99
4. Kim DJ, Lee S, Cheong HJ, Hong S, Kim MJ, Jung SK. Spinal bony involvement of IgG4-related disease treated by a spondylectomy. NMC Case Rep J. 2021. 8: 27-31
5. Lopez A, Abrisqueta P. Plasmablastic lymphoma: Current perspectives. Blood Lymphat Cancer. 2018. 8: 63-70
6. Radotra BD, Aggarwal A, Kapoor A, Singla N, Chatterjee D. An orphan disease: IgG4-related spinal pachymeningitis: Report of 2 cases. J Neurosurg Spine. 2016. 25: 790-4
7. Rumalla K, Smith KA, Arnold PM. Immunoglobulin G4-related epidural inflammatory pseudotumor presenting with pulmonary complications and spinal cord compression: Case report. J Neurosurg Spine. 2017. 26: 688-93
8. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012. 366: 539-51
9. Umehara H, Okazaki K, Kawa S, Takahashi H, Goto H, Matsui S. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol. 2021. 31: 529-33
10. Williams MM, Mashaly H, Puduvalli VK, Jin M, Mendel E. Immunoglobulin G4-related disease mimicking an epidural spinal cord tumor: Case report. J Neurosurg Spine. 2017. 26: 76-80
11. Winkel M, Lawton CD, Sanusi OR, Horbinski CM, Dahdaleh NS, Smith ZA. Neuro-surgical considerations for treating IgG4-related disease with rare spinal epidural compression. Surg Neurol Int. 2018. 9: 209